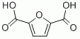
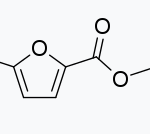
Dimethyl Furan-2,5-dicarboxylate CAS#: 4282-32-0; ChemWhat Code: 622227
Identification
| Product Name | Dimethyl furan-2,5-dicarboxylate |
| IUPAC Name | dimethyl furan-2,5-dicarboxylate |
| Molecular Structure | 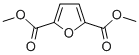 |
| CAS Registry Number | 4282-32-0 |
| Synonyms | FDME, furan-2,5-dicarboxylic acid dimethyl ester, 2,5-furan-dicarboxylic acid dimethyl ester, dimethyl furan-2,5-dicarboxylic acid ester, 2,5-furandicarboxylic acid dimethyl ester, 2-methyl 5-methyl furan-2,5-dicarboxylate, 2,5-furandicarboxylic acid methyl ester, furan-2,5-dicarboxylic dimethyl ester |
| Molecular Formula | C8H8O5 |
| Molecular Weight | 184.149 |
| InChI | InChI=1S/C8H8O5/c1-11-7(9)5-3-4-6(13-5)8(10)12-2/h3-4H,1-2H3 |
| InChI Key | UWQOPFRNDNVUOA-UHFFFAOYSA-N |
| Canonical SMILES | COC(=O)C1=CC=C(O1)C(=O)OC |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN108299357 | Method for preparing disubstituted furan compound | 2018 |
| JP2017/190316 | 2, 5 – furan dicarboxylic acid purification method (by machine translation) | 2017 |
| WO2016/186504 | PROCESS FOR THE PREPARATION OF AN AROMATIC DICARBOXYLIC ACID | 2016 |
| WO2016/202858 | PROCESSES FOR THE FORMATION OF FURANDICARBOXYLIC ACID (FDCA) VIA A MULTISTEP BIOCATALYTIC OXIDATION REACTION OF 5-HYDROXYMETHYLFURFURAL (HMF) | 2016 |
| WO2015/60827 | METHODS AND COMPOUNDS FOR PRODUCING NYLON 6,6 | 2015 |
| US2014/171663 | PROCESS FOR THE PREPARATION OF BENZENE DERIVATIVES FROM FURAN DERIVATIVES | 2014 |
| WO2013/49711 | METHODS FOR PREPARING 2,5-FURANDICARBOXYLIC ACID | 2013 |
| US4327209 | Process for the production of dibenzazolyl compounds | 1982 |
| US2003/176641 | Synthetic ion channels | 2003 |
| US2012/220507 | 2,5-FURAN DICARBOXYLATE DERIVATIVES, AND USE THEREOF AS PLASTICIZERS | 2012 |
| WO2008/9735 | PYRAZOLO (3, 4-B) PYRIDINE DERIVATIVES AS PDE4 INHIBITORS | 2008 |
| US4457924 | 1,1-Alkanediol dicarboxylate linked antibacterial agents | 1984 |
Physical Data
| Appearance | White or off white powder |
| Flash Point | 117.6°C |
| Density | 1.244g/m³ |
| Melting Point, °C | Solvent (Melting Point) |
| 109 – 113 | |
| 112 | chloroform, hexane |
| 110 – 115 | ethyl acetate, hexane |
| 110 | methanol |
| 109 – 110 | ethanol |
| 109 | petroleum ether |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 140 – 145 | 10 |
| 154 – 156 | 15 |
| Description (Electrochemical Characteristics) | Solvent (Electrochemical Characteristics) | Temperature (Electrochemical Characteristics), °C |
| reduction potential | tetrahydrofuran | -74 |
| polarographic current/voltage curve |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Dimethyl Furan-2,5-dicarboxylate CAS#: 4282-32-0 HNMR | 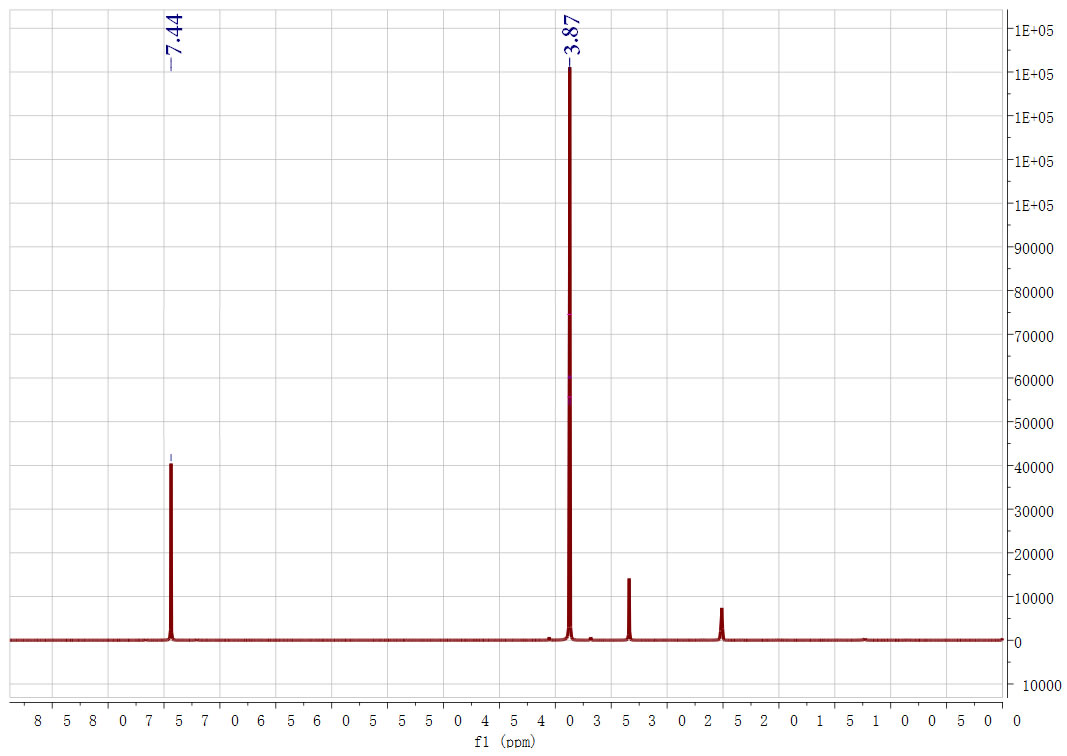 |
| Dimethyl Furan-2,5-dicarboxylate CAS#: 4282-32-0 CNMR | 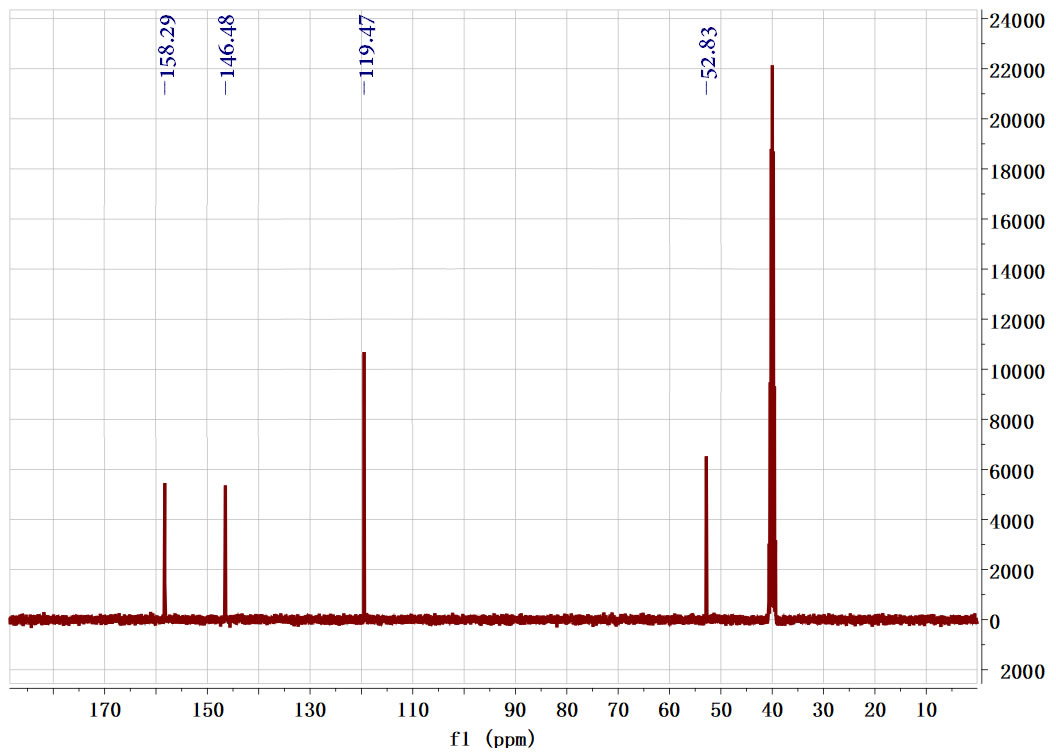 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | |
| Bands | sodium chloride | film |
| Chromatographic data | Original string |
| UPLC (Ultra performance liquid chromatography) | Large spikes at about 4.30 minutes and at 6.059 minutes, respectively, indicate formation of monoester and diesters, with someresidual FDCA at about 2.75 minutes. |
| TLC (Thin layer chromatography) |
Route of Synthesis (ROS)

| Conditions | Yield |
| In methanol at 20℃ under 3450.35 Torr for 19h Experimental Procedure Preparation of dimethyl FDCA from methyl 5-formyl-2- furoate: A 15 niL glass liner was charged with a magnetic stirring bar, methyl 5- formyl-2-furoate (117 mg, 0.76 mmol), methanol (10 mL) and sodium methoxide (4 mg, 0.076 mmol) to give a clear solution. A 1.2 wtpercent Au/Ti02 (41.5 mg, 2.53 μιηο Au) catalyst was added to give a purple suspension and the vial was placed in a 75 mL Parr Hastelloy C-276 reactor. The reactor was closed and flushed 3x with compressed air and then pressurized at 4.6 bar. Stirring was started (600 rpm) and the reaction was allowed to proceed at room temperature. After 19 h, the reaction had consumed 0.25 bar of air and the reactor was opened. The reaction mixture was filtered over Celite to remove the catalyst, which was washed with a httle methanol and dichlorom ethane. The combined organic layers were washed with water, dried over MgS04, filtered, and the solvent was removed under reduced pressure. 2,5-FDCA dimethyl ester was obtained as light yellow crystals (106 mg, yield 76percent). The product was analyzed by H/^C-NMR and GC-MS. Analytical data: NMR (400.17 MHz, CDC13): δ = 7.22 (2 H, s), 3.94 (6 H, s). i3C NMR (100.62 MHz, CDCI3): δ = 52.33, 118.41, 153.86, 158.37. MS (GC-MS, 70eV): m/z (percent) = 184 (32) [M+], 153 (100), 139 (1), 125 (6), 113 (1), 95 (8), 82 (2), 69 (6), 59 (9), 53 (4), 38 (9). | 76% |
| With oxygen In methanol at 20℃ under 3750.38 Torr for 19h |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 520/kg |
| Use Pattern |
| raw material for high-performance epoxy |
| raw material for high-performance polyamide |
| raw material for high-performance polyester |
| raw material for high-performance polyurethane |
| raw material for high-performance resin |
Related Chemicals
Buy Reagent | |
| Sigma-Aldrich APO517178363 | 5g, 25g, 100g, 99% |
| ChemWhat 622227 | 150g, 200g, 250g, 500g, 99% |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Avantium | https://www.avantium.com/ |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |