Dipentaerythritol CAS#: 126-58-9; ChemWhat Code: 58740
Identifiers
| Product Name | Dipentaerythritol |
| IUPAC Name | 2-[[3-hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol |
| Molecular Structure | |
| CAS Registry Number | 126-58-9 |
| Synonyms | 1,3-Propanediol, 2,2′-[oxybis(methylene)]bis[2-(hydroxymethyl)- [ACD/Index Name];2,2′-(oxydimethanediyl)bis[2-(hydroxymethyl)propane-1,3-diol] |
| Molecular Formula | C10H22O7 |
| Molecular Weight | 254.277 |
| InChI | InChI=1S/C10H22O7/c11-1-9(2-12,3-13)7-17-8-10(4-14,5-15)6-16/h11-16H,1-8H2 |
| InChI Key | TXBCBTDQIULDIA-UHFFFAOYSA-N |
| Canonical SMILES | C(C(CO)(CO)COCC(CO)(CO)CO)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN106631778 | Lactic acid polyol ester and its preparation method and in the halogenated vinyl polymer in the processing of the application (by machine translation) | 2017 |
| JP5896028 | A method of manufacturing a polyhydric alcohol ether (by machine translation) | 2016 |
| EP2088163 | CURABLE COMPOSITION CONTAINING HYDROXYL GROUP-CONTAINING THIOL COMPOUND AND CURED PRODUCT THEREOF | 2013 |
| US4540828 | Catalysts for alkoxylation reactions | 1985 |
| US2009/324675 | BIOOCOMPATIBLE POLYMER COMPOSITIONS | 2009 |
| US2003/195373 | Novel beta-hydroxyamides | 2003 |
| US5374366 | Synthetic lubricating oil | 1994 |
Chemical & Physical Properties
| Appearance | White powder |
| Water Solubility | 2.4 g/l at 20 °C (68 °F) – OECD Test Guideline 105 – soluble |
| Melting Point, °C | Solvent (Melting Point) |
| 222 – 225 | H2O |
| 222 | |
| 217 – 224 | acetone |
| 221.7 | H2O |
| 221 | H2O |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.366 | 15 |
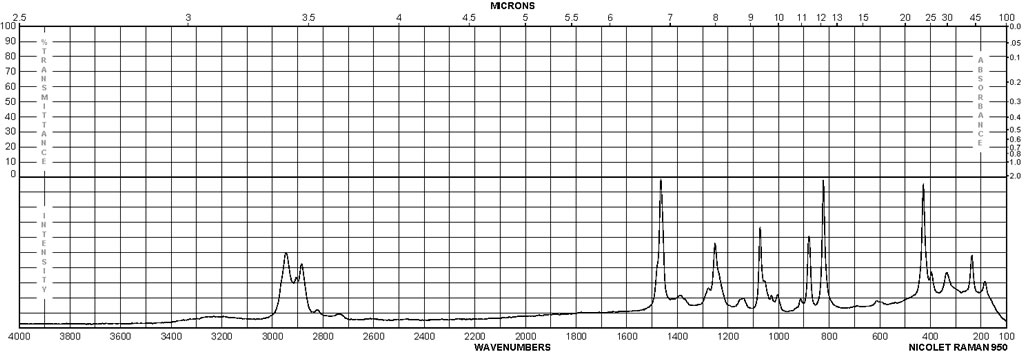
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 250 |
| Chemical shifts | 1H | ||
| Chemical shifts | 13C | D20 |
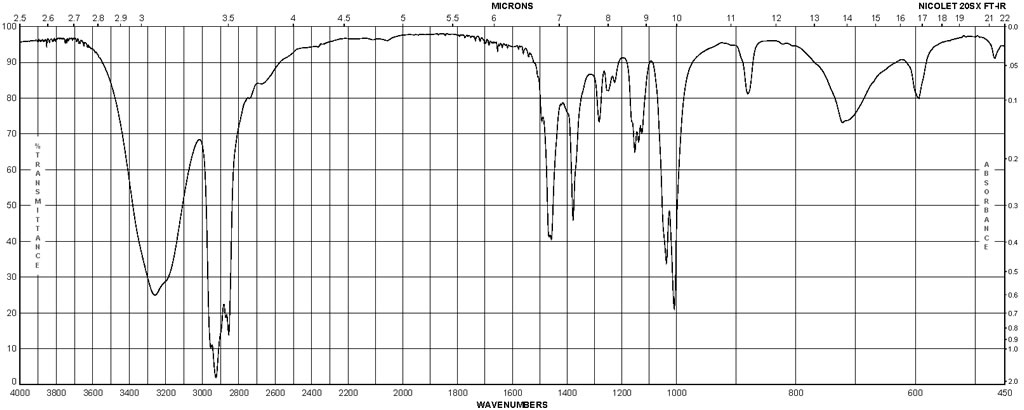
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Spectrum | nujol | 1515 – 699 cm**(-1) |
Route of Synthesis (ROS)
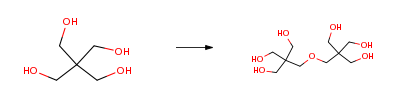
| Conditions | Yield |
| With [1,3]-dioxolan-2-one; tetrabutoxytitanium; boron trioxide; sodium formate at 140 – 190℃ under 412.541 – 727.573 Torr for 14h Reagent/catalyst Pressure Temperature Dean-Stark Experimental Procedure Into a flask equipped with a stirrer, a thermometer, a rectifying column, and a cooling tube,350 parts of PET, 182 parts of ethylene carbonate, titanium tetra-n-butoxide as catalyst B (Trade name: Orgatics TA-25, manufactured by Matsumoto Fine Chemical Co., Ltd.) 0.3 part of diboron trioxide was charged and heated and stirred for 11 hours at a reaction temperature of 140 to 170 °C. and a reaction pressure of 0.055 to 0.0001 MPa. Meanwhile, ethylene glycol and unreacted ethylene carbonate were removed from the system via a rectification column and a cooling pipe. Thereafter, 250 parts of PET, 3.2 parts of diboron trioxide as a catalyst B, and 1.6 parts of sodium formate as a catalyst A were charged in a flask, heated and stirred at a reaction temperature of 190 °C and a reaction pressure of 0.097 MPa for 3 hours. The analysis results of the reaction products are shown in Table 2. | 38% |
| With 2-Ethylhexanoic acid; 2-propyl heptanoic acid at 190 – 231℃ under 150.015 Torr for 26.5h Inert atmosphere Industrial scale Experimental Procedure As an adsorbent, Kyowaad 500 made by Kyowa Chemical Industry Co., Ltd. was used.As the activated carbon, egret P made by Nippon Enviro Chemicals Co., Ltd. was used.82 g of pentaerythritol (0.6 mol, manufactured by K.K. Perstorp), 312 g of 2-ethylhexanoic acid (2.2 mol, manufactured by Kyowa Hakko Kagaku Kabushiki Kaisha) and 124 g of 2-propylheptanoic acid Mole, Production Example), and the mixture was degassed by bubbling nitrogen at room temperature under a reduced pressure of 20 kPa for 30 minutes while stirring the mixture.Then, the mixture was stirred at 190 to 231 ° C for 26 hours under nitrogen bubbling at atmospheric pressure. After the reaction, the reaction product was stirred under a reduced pressure of 0.9 kPa at 228 to 231 ° C for 1.5 hours to distill off the unreacted carboxylic acid in the reaction product. The reaction product was washed with 90 mL of an aqueous solution containing 200 mL of sodium hydroxide having an acid value of 2 times that of the reaction product for 1 hour at 90 ° C. Then, the reaction product was washed with 200 mL of water three times at 90 ° C for 0.5 hours. Then, the reaction product was dried while bubbling nitrogen under a reduced pressure of 0.9 kPa at 105 ° C for 1 hour.1.2 g (corresponding to 0.3percent by weight of the reaction product) of the adsorbent and 4.0 g (1.0percent by weight of the reaction product) of the adsorbent were added to the reaction product, and the reaction product The mixture was stirred at 106 ° C for 1 hour under a reduced pressure of 0.9 kPa and then filtered using a filter aid to obtain 334 g of mixed ester 1. | 334 g |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | 290542 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 9/kg |
Use Pattern
| Wax component in oil in water gel composition as body care formulation |
| It is a component for producing tissue adhesives |
| It is an acrylic monomers for UV curing systems |
| Synthetic lubricants |
| PVC stabilizers and plasticize |
| Rosin esters |
| Alkyd resins |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |