DIPHENYL-2,4,6-TRIMETHYLPHENYLSULFONIUM P-TOLUENESULFONATE CAS#: 347841-51-4; ChemWhat Code: 1490269
Identification
| Product Name | DIPHENYL-2,4,6-TRIMETHYLPHENYLSULFONIUM P-TOLUENESULFONATE |
| IUPAC Name | |
| Molecular Structure | |
| CAS Registry Number | 347841-51-4 |
| EINECS Number | 207-322-2 |
| MDL Number | MFCD00006400 |
| Beilstein Registry Number | 105692 |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | C5H6N2 |
| Molecular Weight | 94.116 |
| InChI | InChI=1S/C5H6N2/c6-5-2-1-3-7-4-5/h1-4H,6H2 |
| InChI Key | CUYKNJBYIJFRCU-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=CN=C1)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP3498694 | NEW BENZAMIDE DERIVATIVES AS PPAR-GAMMA MODULATORS | 2019 |
| WO2019/126730 | CHROMENOPYRIDINE DERIVATIVES AS PHOSPHATIDYLINOSITOL PHOSPHATE KINASE INHIBITORS | 2019 |
| US2018/230157 | PYRROLO[1,2-b]PYRIDAZINE DERIVATIVES | 2018 |
| WO2018/169373 | PYRROLOTRIAZINE DERIVATIVES AS KINASE INHIBITOR | 2018 |
| WO2018/203194 | DIAZABICYCLOOCTANE DERIVATIVES COMPRISING A QUATERNERY AMMONIUM GROUP FOR USE AS ANTIBACTERIAL AGENTS | 2018 |
Physical Data
| Appearance | Light yellow flaky solid |
| Solubility | It is soluble in water as well as soluble in alcohol, benzene. |
| Flash Point | 88 ºC |
| Refractive index | 1.5560 (estimate) |
| Sensitivity | Air Sensitive & Hygroscopic |
| Melting Point, °C | Solvent (Melting Point) |
| 64 | hexane |
| 55 – 57 | ethanol |
| 62 – 63 | aq. ethanol |
| 63 – 64 | benzene, petroleum ether |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.14 | 4 | 25 |
| 1.2 | 4 | -190 |
| 1.24 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | CCl4 | 24.9 | 4-Fluorophenol |
| Stability constant of the complex with … | aq. HNO3 | 25 | AgNO3 |
| Enthalpy of association | acetonitrile | 25 | iodine |
| NMR spectrum of the complex | CDCl3 | Cu(2,4-dichloro-benzoate)2 |
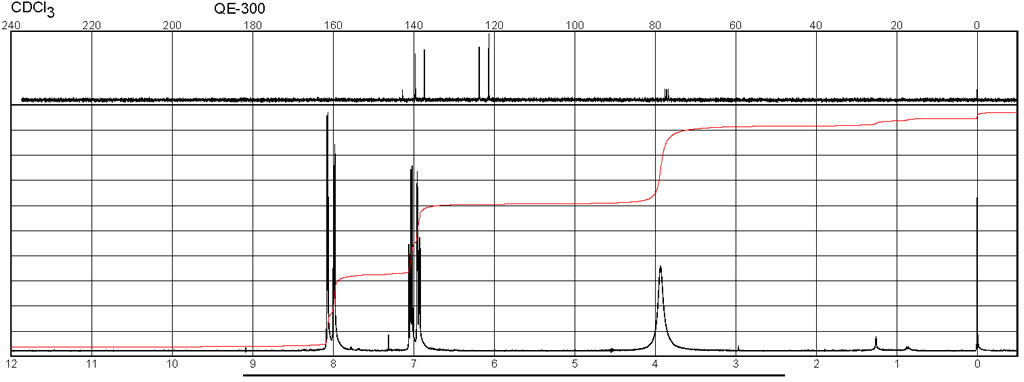
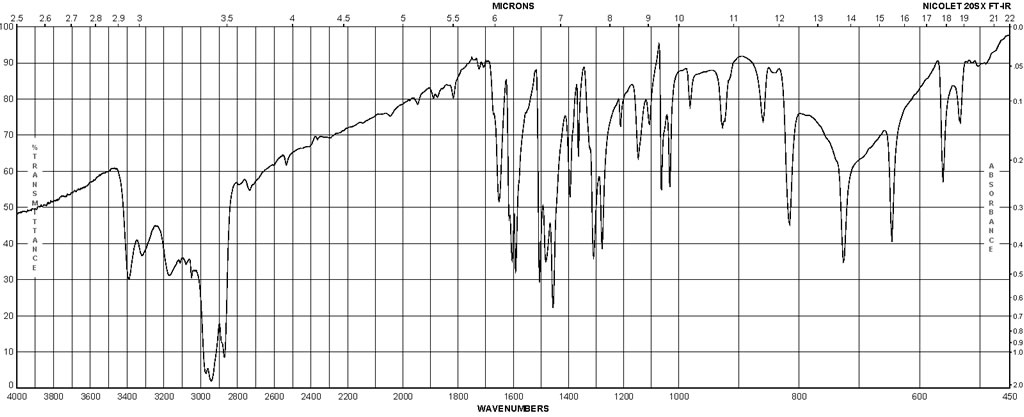
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | 27 |
| Spectrum | CCl4 | 14.85 – 54.85 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | H2O, H2SO4 | Ratio of solvents: 66percent | 258 | 5740 |
| Absorption maxima | H2O, NaOH | Ratio of solvents: 0.1N | 232, 290 | 8600, 3120 |
Route of Synthesis (ROS)

| Conditions | Yield |
| With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 6h; Autoclave; | 99% |
| With 0.2C27H36N2*Pt; hydrogen In tetrahydrofuran at 60℃; under 3000.3 Torr; for 5h; chemoselective reaction; | 99% |
| With hydrogen In ethyl acetate under 760.051 Torr; for 2h; Heating; Flow reactor; Green chemistry; | 99% |
| With hydrogen; triethylamine In ethanol; water at 110℃; under 30003 Torr; for 24h; Autoclave; | 98% |
| With hydrogen In 2-methyltetrahydrofuran; water at 40℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 98% |
| With sodium tetrahydroborate In water at 20℃; for 1.5h; chemoselective reaction; Experimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 97% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H300: Fatal if swallowed [Danger Acute toxicity, oral] H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H331: Toxic if inhaled [Danger Acute toxicity, inhalation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P311, P312, P314, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: II; UN Number: 2671 |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 45/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 94.116 |
| logP | -0.047 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 38.91 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Effect |
| 8.05 | pIC50(Virus replication) | = | 8.05 | antiviral agent | ||
| 8.05 | pIC50 | = | 8.05 | Topoisomerase dna ii 180kda (Beta) [Human immunodeficiency virus 1]:Wild | ||
| 5.65 | EC90(Amount Of P24 Protein) | > | 20 | µM | ||
| 5.48 | IC50(protease activity) | 3.3 | µM | |||
| 4.63 | IC50 | 23.45 | μg | antifungal agent | ||
| 4.59 | IC50 | 2.45 | μg/ml | antifungal agent | ||
| 4.45 | IC50 | 3.33 | μg/ml | antifungal agent | ||
| 4 | stimulation rate | Active | High voltage-activated calcium channel [rat]:Wild | |||
| 3.44 | IC50 | 34.55 | μg/ml | antifungal agent |
| Quantitative Results | ||
| 1 of 10 | Effect | inhibitory activity |
| Target | D-amino-acid oxidase [human]:Wild | |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory concentration of compound against human recombinant D-amino acid oxidase (DAO) expressed in Escherichia coli BL21(DE3)pLysS cells upon incubation in 40 mM sodium pyrophosphate, pH 8.3 for 10 mins at 37 degree C using 10 mM D-Alanine as substrate | |
| 2 of 10 | Assay Description | Clog P value of the compound was calculated using Hansch’s LogP |
| Measurement | Clog P value of the compound | |
| 3 of 10 | Assay Description | Dissociation constant of the compound was measured by using Hammett’s equation |
| Measurement | Dissociation constant | |
| 4 of 10 | Assay Description | Hydrogen bond acidity of the compound was determined |
| Measurement | Hydrogen bond acidity | |
| 5 of 10 | Target | Fatty-acid amide hydrolase 1:Wild |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory actvity of the compound against Fatty acid amide hydrolase in 0.1 M sodium phosphate, pH 8.0 | |
| 6 of 10 | Assay Description | Acid dissociation constant of compound was determined |
| Measurement | Acid dissociation constant | |
| 7 of 10 | Biological material | CEM-T4 cell line |
| Assay Description | Selectivty index of compound was measured as Cytotoxic concentration against mock infected CEM-T4 cells to that of effective concentration required to acieve HIV induced cytopathogenicity | |
| Results | SI50 not calculated | |
| Measurement | SI50 | |
| 8 of 10 | Effect | Genotoxic |
| Biological material | HL-60 cell line | |
| 9 of 10 | Effect | antibiotic agent |
| Biological material | Staphylococcus aureus | |
| Assay Description | Effect : antistaphylococcal | |
| 10 of 10 | Results | effect on phosphatidylcholine secretion in primary cultures of rat type II pneumocytes |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 1 | inhibition rate | 12.5 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | 10 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | CC50 (cytotoxic concentration) | > | 1060 | μM | Cytotoxic |
| 1 | CC90 | > | 1060 | μM | Cytotoxic |
| Use Pattern |
| 3-Aminopyridine CAS#: 462-08-8 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |