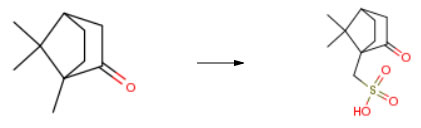
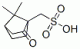DL-10-CAMPHORSULFONIC ACID CAS#: 5872-08-2; ChemWhat Code: 26033
Identification
| Product Name | DL-10-CAMPHORSULFONIC ACID |
| Molecular Structure | 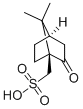 |
| CAS Registry Number | 5872-08-2 |
| EINECS Number | 227-527-0 |
| MDL Number | MFCD00074827 |
| Beilstein Registry Number | 3205973 |
| Synonyms | camphor-10-sulfonic acid, DL-10-camphorsulphonic acid, (7,7-dimethyl-2-oxobicyclo(2,2,1)heptan-1-yl)methanesulfonic acid, (7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid, 7,7-dimethyl-2-oxobicyclo[2.2.1]-heptane-1-methanesulfonic acid, 10-camphorsulfonic acid||camphor-10-sulfonic acid||CSA||10-CSA, (±)-camphor-10-sulfonic acid |
| Molecular Formula | C10H16O4S |
| Molecular Weight | 232.301 |
| InChI | InChI=1S/C10H16O4S/c1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h7H,3-6H2,1-2H3,(H,12,13,14)/t7-,10+/m1/s1 |
| InChI Key | MIOPJNTWMNEORI-UHFFFAOYSA-N |
| Canonical SMILES | CC1(C2CCC1(C(=O)C2)CS(=O)(=O)O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2008/145605 | IMPROVED SYNTHESIS OF (2S-CIS)-2-(BROMOMETHYL)-2-(4-CHLOROPHENYL)-1,3 DIOXOLANE-4-METHANOL METHANESULFONATE(ESTER) | 2008 |
| US2004/24254 | Process for preparation of optically active halogeno hydroxypropyl compound and glycidyl compound | 2004 |
| US6348622 | Vitamin a related compounds and process for producing the same | 2002 |
| US6372945 | Process for the synthesis of vinyl sulfoxides | 2002 |
Physical Data
| Appearance | White or almost white crystalline powder |
| Melting Point | 193~202 °C |
| Flash Point | 207.324°C |
| Boiling Point | 344.46°C (rough estimate) |
| Density | 1.2981 (rough estimate) |
| Water Solubility | Soluble in water |
| Refractive index | 1.5400 (estimate) |
| Description (Association (MCS)) | Comment (Association (MCS)) | Partner (Association (MCS)) |
| IR spectrum of the complex | film | N,N-dimethyl-formamide |
| Further physical properties of the complex | polyacrylic acid, polyaniline; Monomer(s): aniline | |
| Further physical properties of the complex | polyaniline |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | chloroform-d1 | 400 | |
| Spectrum | 13C | [D3]acetonitrile | 26.84 | 126 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sulfuric acid; acetic anhydride |
Other Data
| Transportation | UN number: 3261; Class: 8; Packing group: II |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 60/kg |
| Use Pattern |
| Camphor-10-sulfonic acid (β) (CSA) is extensively used as an acid catalyst. • It can be used in a catalytic amount to protect hydroxyl groups as tetrahydropyranyl (THP) ethers using dihydropyran. • It also catalyzes the protection of ketones as ketals. • It is a useful catalyst for the intramolecular ring opening of epoxides. • CSA can also be used to catalyze nucleophile-promoted alkyne-iminium cyclization in the total synthesis of pumiliotoxin A. |
| starting material for manufacturing a composite catalyst suitable for making polyethylene terephthalate resin |
| Resolving agent |
| Camphorsulfonic acid is a organosulphur compound. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |