DOCOSAHEXAENOIC ACID CAS#: 25167-62-8(6217-54-5); ChemWhat Code: 248074
Identification
| Product Name | DOCOSAHEXAENOIC ACID |
| IUPAC Name | (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid |
| Molecular Structure | 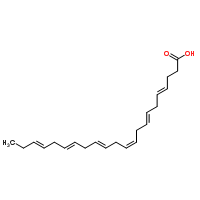 |
| CAS Registry Number | 25167-62-8 (6217-54-5) |
| EINECS Number | No data available |
| MDL Number | MFCD00065722 |
| Beilstein Registry Number | 1715505 |
| Synonyms | 25167-62-8(6217-54-5);Docosahexaenoic acid;DHE;4,7,10,13,16,19-Docosahexaenoate 4,7,10,13,16,19-docosahexaenoic acid;all-Z-Docosahexaenoate;all-Z-Docosahexaenoic acid Cervonate;Cervonic acid;cis-4,7,10,13,16,19;Docosahexanoate;cis-4,7,10,13,16,19-Docosahexanoic acid |
| Molecular Formula | C22H32O2 |
| Molecular Weight | 328.49 |
| InChI | InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- |
| InChI Key | MBMBGCFOFBJSGT-CHRIZAQASA-N |
| Canonical SMILES | CCC=CCC=CCC=CCC=CCC=CCC=CCCC(=O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/197086 | PROCEDURE FOR THE OBTAINMENT OF FATTY ACIDS OF PHARMACOLOGICAL AND NUTRITIONAL INTEREST | 2013 |
| WO2006/117675 | FATTY ACID-BENZENEDIOL DERIVATIVES AND METHODS OF MAKING AND USING THEREOF | 2006 |
| WO2005/73164 | THERAPEUTIC AND CARRIER MOLECULES | 2005 |
| WO2005/12316 | METHOD FOR THE PRODUCTION OF MULTIPLY-UNSATURATED FATTY ACIDS IN TRANSGENIC ORGANISMS | 2005 |
| EP1408030 | NOVEL ALIPHATIC COMPOUND, METHOD OF SYNTHESIS, AND METHOD OF UTILIZATION | 2004 |
Physical Data
| Appearance | Light yellow liquid |
| Flash Point | 62°C |
| Refractive index | 1.5030-1.5060 |
| Sensitivity | Light and Air Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 60 – 62 | |
| -47.4 – -42.2 | |
| -44 | |
| -44.5 – -44.1 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5043 | 589 | 20 |
| 1.5017 | 589 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 |
| 1H | chloroform-d1 | 300 | |
| 1H | chloroform-d1 | 400 | |
| Spin-lattice relaxation time (T1), Chemical shifts | 13C | benzene-d6 | |
| Chemical shifts | 1H | CDCl3 | 250 |
| Spin-spin coupling constants | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide | |
| Bands | neat (no solvent, solid phase) | |
| Intensity of IR bands, Bands, Spectrum | potassium bromide | |
| Bands | neat (no solvent) | 3013 – 730 cm**(-1) |
| Bands | NaCl | 1710 – 1645 cm**(-1) |
| Spectrum | CCl4 | 5000 – 833 cm**(-1) |
| Spectrum | CS2 | 833 – 667 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Log epsilon |
| methanol | 210.5 | 0.9 | ||
| 279.6 | ||||
| UV/VIS | 258 | |||
| Spectrum | 210-240nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| With ethylenediaminetetraacetic acid; edetate disodium In ethanol; water at 60 – 65℃; for 2h; | 98% |
| With sodium hydroxide; edetate disodium In ethanol at 65℃; | 98% |
| Stage #1: all-(Z)-ethyl 4,7,10,13,16,19-docosahexaenoate With water; sodium hydroxide In methanol at 20℃; for 1.5h; Stage #2: With hydrogenchloride In methanol; water Cooling with ice; Experimental Procedure Cis-docosahexaenoic acid (DHA): To a solution of ethyl cis-docosahexaenoate (5 g, 14.0 mmol) in methanol (100 mL) was added an aqueous solution of NaOH (6 g, 150 mmol, in 20 mL of water) at rt and stirred for 1.5 h. The reaction mixture was poured into ice cooled water and acidified with dil. HCl. The solution was extracted with DCM (3 x 50 mL) and the combined layer was washed with water, brine and dried over sodium sulfate. The solution was filtered and the solvent evaporated to give the acid (4.2 g, 91%) as an oil; LCMS (ESI, negative ion mode): m/z 327 (M-H)“. | 91% |
| With lithium hydroxide In ethanol; water Ambient temperature; | |
| With lithium hydroxide at 20℃; | |
| With lithium hydroxide monohydrate In ethanol; water at 20℃; for 4h; Inert atmosphere; Experimental Procedure Dihydro-5-((3Z,6Z,9Z,12Z,15Z)-1-iodooctadecapentaenyl)furan-2(3H)-one (8) A mixture of DHA ethyl ester (10.02 g, 28 mmol) and LiOH.H2O (5.8 g, 140 mmol) in EtOH-H2O (1:1) (60 mL) was left stirring until all the DHA ethyl ester was converted (TLC, CH2Cl2). Water (90 mL) were added, the reaction flask was covered with aluminium-foil and cooled to 0 °C. Hydrogen iodide (57%; 20 mL) was added to the reaction mixture, followed successively by saturated KHCO3 (10 mL) and dropwise addition of a solution of I2 (21.32 g, 84 mmol) in EtOH (70 mL). The mixture was left stirring at 0-4 °C in the dark for 18 h. The reaction was quenched by adding a saturated aq. solution of Na2S2O3 (100 mL). Solid NaCl was added to saturation and the product extracted with hexane (3 × 50 mL). The extract was washed with brine (2 × 50 mL), dried (Na2SO4) and evaporated under reduced pressure to give 8 (12.3 g; 97%) as pale yellow oil. Spectral data were in agreement with those previously reported [17]. |
Safety and Hazards
| Pictogram(s) | No data available |
| Signal | No data available |
| GHS Hazard Statements | No data available |
| Precautionary Statement Codes | No data available |
Other Data
| Transportation | Class 3; Packaging Group: II; UN Number: 1170 |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Use Pattern |
| DOCOSAHEXAENOIC ACID CAS#: 25167-62-8(6217-54-5) as Pharmaceuticals |
| DOCOSAHEXAENOIC ACID CAS#: 25167-62-8(6217-54-5) as active ingredient of lipid extract of New Zealand green-lipped mussel composition for treating osteoporosis |
| for increasing bone mineral density in a patient |
| CFU-F-forming ability |
| angiogenesis-promoting ability |
| anti-inflammatory immunosuppression-inducing function |
| nutritional formula in combination with alpha-lactalbumin enriched whey protein concentrate, oleic acid-palmitic acid-oleic acid triglyceride, lactose, lutein, lactoferrin, arachidonic acid, galactooligosaccharides, polydextrose, oseteopontin, beta-casein enriched milk protein, mildly hydrolyzed milk protein, linoleic acid, alpha-linolenic acid, vitamins, minerals, nucleotides, L-choline bitartarate, L-carnitine, soya lecithin, beta-carotene, taurine |
| promoting postnatal development of an infant’s gastrointestinal functions |
| inhibitor of ER stress |
| mitigating in a subject one or more symptoms associated with a disease characterized by alpha-synuclein aggregates or deposits in the brain, or delaying or preventing the onset of said symptoms, in combination with inhibitor of cyclooxygenase-2 (COX-2), inhibitor of phosphodiesterase and inhibitor of soluble epoxide hydrolase (sEHI) |
| upregulating bone morphogenetic protein 4 (BMP4) |
| in combination with vitamin B12, a nitric oxide releasing compound |
| support or maintenance of a healthy brain |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | http://www.ulcho.com/ |
| AK&MN BioFarm | http://www.akb.co.kr/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |