(E,E)-2,4-Hexadienal CAS#: 142-83-6; ChemWhat Code: 74692
Identification
| Product Name | (E,E)-2,4-Hexadienal |
| IUPAC Name | (2E,4E)-hexa-2,4-dienal |
| Molecular Structure | 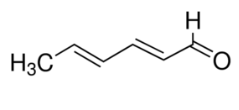 |
| CAS Registry Number | 142-83-6 |
| EINECS Number | 205-564-3 |
| MDL Number | MFCD00007004 |
| Beilstein Registry Number | 1698401 |
| Synonyms | trans,trans-2,4-Hexadienal, 2,4-Hexadienal, trans,trans-2,4-hexadienal;CAS Number: 142-83-6 |
| Molecular Formula | C6H8O |
| Molecular Weight | 96.12 |
| InChI | InChI=1S/C6H8O/c1-2-3-4-5-6-7/h2-6H,1H3/b3-2+,5-4+ |
| InChI Key | BATOPAZDIZEVQF-MQQKCMAXSA-N |
| Canonical SMILES | C/C=C/C=C/C=O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2018/305276 | METHOD FOR PRODUCING 2,4-DIENAL ACETAL COMPOUND AND 2,4-DIENAL COMPOUND | 2018 |
| CN106256816 | A α, β – unsaturated carbonyl compound isomer E – Z – isomer of preparation (by machine translation) | 2016 |
| EP2832233 | 1H-pyrrole-2,4-dicarbonyl-derivatives and their use as flavoring agents | 2015 |
| WO2003/95403 | POLYUNSATURATED LINEAR ALDEHYDES AND THEIR DERIVATIVES WITH ANTI-RADICAL AND ANTI-TUMORAL ACTIVITY | 2003 |
| US4073813 | Process for the preparation of unsaturated alcohols | 1978 |
Physical Data
| Appearance | Yellow to red liquid |
| Solubility | It is Insoluble in water, but soluble in ethanol. |
| Boiling Point | 69 °C20 mm Hg(lit.) |
| Refractive index | 1.5560 (estimate) |
| Sensitivity | Air Sensitive |
| Melting Point, °C |
| -18 |
| -17.5 – -16.5 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 60 | |
| 75 – 76 | 30 |
| 33 – 34 | 0.3 |
| 68 – 68.5 | 20 |
| 47 – 47.5 | 7 |
| 173 – 174 | 754 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 173 – 174 | 20 | 20 |
| 0.9087 | 0 | 22 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5346 | 20 | |
| 1.5391 | 589 | 20.5 |
| 1.5367 | 589 | 27 |
| 1.5425 | 589 | 20 |
| 1.5372 | 589 | 22 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | ||
| Chemical shifts | 1H | chloroform-d1 | 500 | 1H-NMR (500 MHz, CDCl3): δ=1.92 (3H, d, J=5.2 Hz), 6.06 (1H, dd, J=7.9, 15.4 Hz), 6.18-6.44 (2H, m), 7.00-7.16 (1H, m), 9.54 (1H, d, J=7.9 Hz) ppm | |
| Chemical shifts | 1H | 399.9 | |||
| Chemical shifts | 1H | CDCl3 | 400 | ||
| 1H | 1H | CDCl3 | 400 | ||
| Chemical shifts | 13C | CDCl3 | 100 | ||
| Spectrum | 1H | CDCl3 | 400 | ||
| Chemical shifts | 1H | CD2Cl2 | |||
| Chemical shifts | 13C | pentane | |||
| Chemical shifts | 13C | triethylamine | |||
| Chemical shifts | 13C | dimethylsulfoxide-d6 | |||
| Chemical shifts | 1H | CD3CN |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat liquid | |
| Bands | KBr | 1682 cm**(-1) |
| Spectrum | neat (no solvent) | 3100 – 50 cm**(-1) |
| Bands | CCl4 | 3035 – 298 cm**(-1) |
| Bands | neat (no solvent) | 1680 – 1600 cm**(-1) |
| Spectrum | CHCl3 | 2000 – 1429 cm**(-1) |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), IT (ion trap), spectrum |
| spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | gas | |||
| Absorption cross-section | gas | |||
| Absorption maxima | ethanol | 271 | ||
| Spectrum | various solvent(s) | 500-200nm | ||
| Spectrum | diethyl ether, 2-methyl-butane | 500 – 200 nm, Ratio of solvents: 5:5:2(alcohol) | ||
| Absorption maxima | various solvent(s) | 253 | ||
| Absorption maxima | diethyl ether, 2-methyl-butane | Ratio of solvents: 5:5:2(alcohol) | 267 | 31900 |
| Absorption maxima | 2,2,4-trimethyl-pentane | 261 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With oxygen; Pd561phen60(OAc)180 In acetic acid at 60℃; for 2h; | 100% |
| With (1,10-phenanthrolino)2-tetrapalladium(CO)(acetate)4; oxygen In benzene at 50℃; for 24h; | 96% |
| With 1,4-diaza-bicyclo[2.2.2]octane; 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; oxygen; 1,3-di(4-pyridyl)propane; copper(II) perchlorate In dimethyl sulfoxide at 20℃; under 760 Torr; for 2h; | 85% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H226 (100%): Flammable liquid and vapor [Warning Flammable liquids] H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H311 (100%): Toxic in contact with skin [Danger Acute toxicity, dermal] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P270, P272, P280, P301+P312, P302+P352, P303+P361+P353, P312, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P370+P378, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1(8); Packaging Group: III; UN Number: 2922 |
| Under the room temperature and away from light | |
| HS Code | 291219 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 96.1289 |
| logP | 1.511 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 17.07 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target |
| 10.5 | amount | 30 – 60 | pM | ||
| 4.28 | activation percentage(relative to standard agonist) | 108.71 | % | Transient receptor potential cation channel subfamily V member 1 [human]:WildTransient receptor potential cation channel subfamily A member 1 [human]:Wild | |
| 4.06 | activation percentage(relative to standard agonist) | 92 | % | Transient receptor potential cation channel subfamily A member 1 [human]:Wild | |
| 4.02 | Km (Michaelis constant)(Michaelis-Menton constant) | 95.7 | µM | Aldo-keto reductase family 1 member B10:Wild | |
| kcat(Catalytic constant) | = | 82.8 | min-1 | Aldo-keto reductase family 1 member B10:Wild | |
| score(stimulation of CCK secretion) | = | 0.84 | no unit |
| Quantitative Results | ||
| 1 of 8 | Target | Aldo-keto reductase family 1 member B10:Wild |
| Biological material | HEK293T cell line | |
| Assay Description | Maximum velocity of the compound towards recombinant EGFP-aldo-keto reductase family 1 B10 protein transfected into 293T cell line upon incubation in 135mM sodium phosphate (pH 7.0), 0.2mM NADPH, 1.0mM beta-mercaptoethanol, 50mM KCl at 35 degree C for 20 min | |
| Results | 1 | |
| 2 of 8 | Target | Aldo-keto reductase family 1 member B10:Wild |
| Biological material | HEK293T cell line | |
| Assay Description | Ratio of catalytic constant of the compound to that of Michaelis-Menton constant towards aldo-keto reductase family 1 B10 protein | |
| Results | 1 | |
| 3 of 8 | Biological material | human HL-60 cell line |
| Assay Description | Effect : DNA adduct formation Bioassay : in vitro; RPMI 1640 medium; incub. in 5 percent CO2 atmosphere at 37 deg C for 4 h; cells incub. with or without NaBH4 on ice for 30 min; DNA-protein cross-link (DNAPC) levels determ. | |
| 4 of 8 | Effect | Cytotoxic |
| Assay Description | Target : Chinese hamster lung V79 fibroblasts Bioassay : cells incub. with title comp. for 1 h; cell suspension was mixed with tryphan blue solution and membrane integrity was examined microscopically | |
| Results | cytotoxicity (loss of membrane integrity) of cells was <15% in all incubation/post-incubation of title comp. | |
| 5 of 8 | Effect | Cytotoxic |
| Biological material | human Caco-2 cell line | |
| Assay Description | Bioassay : cells incub. with title comp. for 1 h; cell suspension was mixed with tryphan blue solution and membrane integrity was examined microscopically | |
| Results | viability (membrane integrity) of cells was >85% in all incubation/post-incubation of title comp. | |
| 6 of 8 | Effect | Genotoxic |
| Assay Description | Target : Chinese hamster lung V79 fibroblasts Bioassay : control: 0.1% DMSO; DNA migration is directly expressed as mean tail intensity (TI%); FPG: formamidopyrimidine-DNA glycosylase cells incub. with title comp. for 1 h; centrifuged, mixed with low melting agarose on slides; lysed; covered with FPG; alkaline single cell gel electrophoresis was performed; DNA damage was determined by comet assay | |
| Results | title comp. at 300 μmol/l caused distinct direct DNA breakage (TI% > 20) and FPG-sensitive sites became apparent, whereas at 100 μmol/l not effective; at 100 μmol/l FPG-sensitive sites was not observed; fig. | |
| 7 of 8 | Effect | Oxidant |
| Assay Description | Target : Chinese hamster lung V79 fibroblasts Bioassay : control: without title comp.; DTNB: NADPH/5.5′-dithiobis(2-nitribenzoic acid); tGSH: total glutathione (GSH); TNB: 5-thio-2-nitrobenzoate cells incubated with title comp. for 1 h at 37 deg C in incubation medium; centrifuged; DTNB solution added to supernatant; after addn. of glutathione reductase, TNB formation rate measured at 412 nm; tGSH determined by photometric determination of TNB | |
| Results | title comp. induced strong depletion of GSH levels to <20% of control during 1 h incubation; title comp. pretreated cells showed increase in tGSH (>30% of control) during 3 h post-incubation; fig. | |
| 8 of 8 | Assay Description | Effect : biotransformation Target : CD-1 mouse liver microsomes Bioassay : in vitro; metabolism of title comp. tested; 50 μmol/l FeSO4; 2.0 mmol/l NADPH; phosphate buffer,pH 7.4; 37 deg C; incubated for 10 min; also in the presence of pyrazole or cyanamide; metabolites quantitated by HPLC |
| Results | pyrazole significantly inhibited title comp. monoreduction and monooxidation; cyanamide did not decrease title comp. metabolism significantly |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit |
| 4.7 | concentration (parameter)(LED) | = | 1.9 | % |
| 1 | number(Number of deaths) | Not active | ||
| number(Number of deaths) | Active | |||
| LD50(Lethal dose) | = | 0.27 | mL/kg | |
| LD50 | = | 0.3 | g/kg | |
| number(Number of deaths) | Active | |||
| = | 74 | µg/mL | ||
| = | 2.5 | μM |
| Qualitative Results | ||
| 1 of 3 | Biological material | Tetrahymena pyriformis |
| Assay Description | Toxic activity of compound in tetrahymena pyriformis | |
| 2 of 3 | Biological material | Salmonella enterica serovar Typhimurium |
| Assay Description | Mutagenicity in Salmonella typhimurium, in presence and absence of hamster liver S9 homogenate; 100-10000 ug/plate; Negative;AMES test | |
| Results | Mutagenicity not calculated | |
| Measurement | Mutagenicity | |
| 3 of 3 | Effect | Cytotoxic |
| Biological material | human HL-60 cell line | |
| Assay Description | Effect : cell viability Bioassay : in vitro; RPMI 1640 medium; incub. in 5 percent CO2 atmosphere at 37 deg C for 2-4 h; cell viability assessed by trypan blue exclusion assay |
| Use Pattern |
| (E,E)-2,4-Hexadienal CAS#: 142-83-6 as Food/food additives |
| (E,E)-2,4-Hexadienal CAS#: 142-83-6 in combination with 2,4-hexadien-1-ol |
| (E,E)-2,4-Hexadienal CAS#: 142-83-6 in combination with 2-methylbutanol |
| (E,E)-2,4-Hexadienal CAS#: 142-83-6 in combination with 3-methylbutanol |
| in combination with butyl acetate |
| in combination with cis-4- methyl-5-butyldihydro-2(3H)-furanone |
| in combination with trans-4-methyl-5-butyldihydro-2(3H)-furanone |
| (E,E)-2,4-Hexadienal CAS#: 142-83-6 used as an appetite-suppressing food product |
| cholecystokinin secretion-promoting composition |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |