EPA CAS#: 10417-94-4; ChemWhat Code: 34144
Identification
| Product Name | EPA |
| IUPAC Name | (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoic acid |
| Molecular Structure | 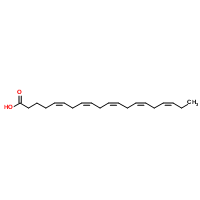 |
| CAS Registry Number | 10417-94-4 |
| MDL Number | MFCD00065716 |
| Beilstein Registry Number | 1714433 |
| Synonyms | all cis-5,8,11,14,17-eicosapentaenoic acid, eicosapentaenoic acid |
| Molecular Formula | C20H30O2 |
| Molecular Weight | 302.451 |
| InChI | InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- |
| InChI Key | JAZBEHYOTPTENJ-JLNKQSITSA-N |
| Canonical SMILES | CCC=CCC=CCC=CCC=CCC=CCCCC(=O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/59801 | FATTY ACID AMIDES, COMPOSITIONS AND METHODS OF USE | 2013 |
| US2014/99354 | BIOPASSIVATING MEMBRANE STABILIZATION BY MEANS OF NITROCARBOXYLIC ACID-CONTAINING PHOSPHOLIPIDS IN PREPARATIONS AND COATINGS | 2014 |
| US2014/148464 | SALT FORMS OF FATTY ACIDS FOR THE TREATMENT OF DRY EYE | 2014 |
| US2015/119409 | COMPOSITIONS AND METHODS FOR THE TREATMENT OF INFLAMMATORY DISORDERS | 2015 |
| US2013/197086 | PROCEDURE FOR THE OBTAINMENT OF FATTY ACIDS OF PHARMACOLOGICAL AND NUTRITIONAL INTEREST | 2013 |
| US2006/229461 | Method for preparing unsaturated fatty acids | 2006 |
| WO2006/117675 | FATTY ACID-BENZENEDIOL DERIVATIVES AND METHODS OF MAKING AND USING THEREOF | 2006 |
| WO2005/73164 | THERAPEUTIC AND CARRIER MOLECULES | 2005 |
| WO2005/12316 | METHOD FOR THE PRODUCTION OF MULTIPLY-UNSATURATED FATTY ACIDS IN TRANSGENIC ORGANISMS | 2005 |
Physical Data
| Appearance | Clear,Colourless to pale yellow liquid |
| Solubility | Soluble in methanol |
| Flash Point | 93 °C |
| Refractive index | n20/D 1.4977(lit.) |
| Sensitivity | Air & Light Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 52 – 54 | |
| -54 | |
| -54.4 – -53.8 | |
| 63 – 64 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.4965 | 589 | 20 |
| 1.4968 | 589 | 20 |
| 1.489 | 589 | 22 |
| 1.4977 | 589 | 23 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.9284 | 4 | 20 |
| log POW | pH | Comment (Partition octan-1-ol/water (MCS)) |
| 6.73 | ||
| 6.73 | 7.4 | aq.buffer |
| 6.8 |
| Solubility, g·l-1 | Saturation | Solvent (Association (MCS)) |
| 0.2 | water | |
| 0.3 | in pure solvent | water |
| 0.3 | in pure solvent | water |
| 0.2 | water |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Comment (NMR Spectroscopy) |
| Chemical shifts | 13C | |||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | d(4)-methanol | 500 | |
| 1H | Signals given | |||
| 13C | Signals given | |||
| Chemical shifts | 1H | tetradeuteriomethanol | ||
| Chemical shifts | 13C | tetradeuteriomethanol | 75 | |
| Chemical shifts | 1H | CDCl3 | 250 | |
| 1H | CDCl3 | 250 | ||
| Chemical shifts | 13C | CDCl3 | 62.5 | |
| Spin-lattice relaxation time (T1), Chemical shifts | 13C | benzene-d6 | ||
| Chemical shifts | 1H | CDCl3 | ||
| Spin-spin coupling constants | CDCl3 | 1H-1H |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent, solid phase) | |
| Bands | 1709 cm**(-1) | |
| Bands | neat (no solvent) | 3000 – 720 cm**(-1) |
| Spectrum | CS2 | 5000 – 714 cm**(-1) |
| Spectrum | CCl4 | 5000 – 714 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Log epsilon |
| methanol | 212.9 | 1.1 | |
| 256.5 | |||
| UV/VIS |
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Spectrum, Bands |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Intensity, % |
| electrospray ionisation (ESI), spectrum | ||
| liquid chromatography mass spectrometry (LCMS), time-of-flight mass spectra (TOFMS), electrospray ionisation (ESI), spectrum | ||
| tandem mass spectrometry, spectrum | ||
| time-of-flight mass spectra (TOFMS), spectrum | ||
| electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), time-of-flight mass spectra (TOFMS), spectrum | ||
| high resolution mass spectrometry (HRMS), liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum | ||
| gas chromatography mass spectrometry (GCMS), time-of-flight mass spectra (TOFMS), spectrum |
Route of Synthesis (ROS)
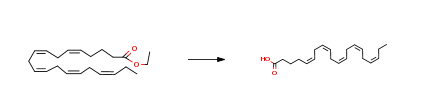
| Conditions | Yield |
| With ethylenediaminetetraacetic acid; edetate disodium In ethanol; water at 60 – 65℃; for 2h; | 98% |
| With immobilized lipase from Candida Antarctica; water In 1,4-dioxane at 55℃; for 3h; Experimental Procedure To a solution of the ethyl ester of EPA (200mg, 0.6mmol) in 1.7mL of dioxane, 300µL of distilled water and Novozym 435 (200mg) were added. The mixture was stirred at 55°C and 280rpm in a shaker for 3h. Then, the enzyme was separated by filtration and the solution taken to dryness under reduced pressure. The residue was purified by column chromatography (Si gel 3.9 g, from n-hexane to n-hexane/EtOAc 9:1 containing 0.1% of HCOOH, 200mL) to give pure (TLC analysis) EPA (180mg, 0.59mmol, 98% yield) as a colorless oil. | 98% |
| With Candida antarctica lipase; water at 40℃; Hydrolysis; | 97.6% |
| With sodium hydroxide; edetate disodium In ethanol at 65℃; | 97% |
| Stage #1: eicosapentaenoic acid ethyl ester With water; sodium hydroxide In methanol at 20℃; for 1.5h; Stage #2: With hydrogenchloride In methanol; water Cooling with ice; | 96% |
| With sodium hydroxide In ethanol; water at 20℃; for 2h; Experimental Procedure (5Z,8Z,11Z,14Z,17Z)-ethyl icosa-5,8,11,14,17-pentaenoate (4, 10 g, 00302 mol, 1 eq.) was dissolved(200 ml) and added NaOH aqueous solution (11.98mol, 9.9 eq., 50 ml of H20). Then the Reaction Mixturestirred for 2 h at room temperature. After completionreaction, volatiles were evaporated under reducedrota evaporatot The obtained crude materialwith water (50 ml) and acidified with 3Nwith ethyl acetate (2×300 ml). The combinedextracts were washed with brine (2×100 mE), driedanhydrous sodium sulfate, filtered and concentratedPurification on silica gel (20% EtOAc in hexane)compound 5 (9 g, 90% yield); Mass (m/z): 301 (M-H). | 90% |
| With water; sodium hydroxide In methanol at 20℃; for 4h; Concentration; | 87.4% |
| With sodium hydroxide In methanol; water at 20℃; for 2h; | 83.12% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class: 8; Packaging Group: III; UN Number: 3265 |
| Under the room temperature and away from light | |
| HS Code | 291619 |
| Storage | Stored in refrigerator, the product is very air sensitive and easily oxidized by air, so do always keep the product under protection of nitrogen. |
| Shelf Life | 1 year |
| Market Price | USD |
| Use Pattern |
| Pharmaceuticals |
| EPA CAS# 10417-94-4 as active ingredient of lipid extract of New Zealand green-lipped mussel composition for treating osteoporosis |
| EPA CAS# 10417-94-4 for reducing a patient’s risk for developing osteoporosis |
| CFU-F-forming ability |
| angiogenesis-promoting ability |
| anti-inflammatory immunosuppression-inducing function |
| inhibits NOD2 |
| upregulating bone morphogenetic protein 4 (BMP4) |
| reducing or preventing oxidative modification of membrane polyunsaturated fatty acids |
| gastrointestinal stromal tumors (GIST) |
| identifying ALDH mnutation or deletion in a subject |
| inhibiting progression of neurodegenerative disease associated with a mutated RDH protein |
| preventing, ameliorating or inhibiting progression of Liebers Congenital Amurosis |
| EPA CAS# 10417-94-4 is reducing fecal calprotectin (FC) level in combination with another therapeutic agent and pharmaceutically acceptable carrier |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| AK&MN BioFarm | http://www.akb.co.kr/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
