Ethylene glycol CAS#: 107-21-1; ChemWhat Code: 25030
Identification
| Product Name | Ethylene glycol |
| IUPAC Name | ethane-1,2-diol |
| Molecular Structure |  |
| CAS Registry Number | 107-21-1 |
| EINECS Number | 203-473-3 |
| MDL Number | MFCD00002885 |
| Beilstein Registry Number | 505945 |
| Synonyms | ethylene glycol, 1,2-ethanediol;CAS 107-21-1; CAS NO,: 107-21-1;CAS number : 107-21-1 |
| Molecular Formula | HOCH2CH2OH |
| Molecular Weight | 62.07 |
| InChI | InChI=1S/C2H6O2/c3-1-2-4/h3-4H,1-2H2 |
| InChI Key | LYCAIKOWRPUZTN-UHFFFAOYSA-N |
| Canonical SMILES | C(CO)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110862293 | Continuous method for preparing dihalogenated alkane by using glycol compound (by machine translation) | 2020 |
| CN111004125 | Preparation method of acetal or ketal compound (by machine translation) | 2020 |
| US2020/123094 | METHOD FOR PRODUCING CINNAMIC ACID ESTER COMPOUND | 2020 |
| CN111217856 | Pentacyclic phosphate compound as well as preparation method and application thereof (by machine translation) | 2020 |
| WO2020/86271 | PROCESSES FOR FORMING GLYCOLS | 2020 |
Physical Data
| Appearance | Clear Colourless Liquid |
| Solubility | water: miscible |
| Flash Point | 88 ºC |
| Refractive index | n20/D 1.431(lit.) |
| Sensitivity | Hygroscopic |
| Melting Point, °C | Solvent (Melting Point) |
| -16 | |
| -11.58 | |
| -12.65 | stabile Form. |
| -13.9 | metastabile Form. |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 80 | 0.2 |
| 197.3 | |
| 196 – 198 | |
| 197.5 | 760.051 |
| 195 | 760.051 |
| 80 | 1.9502 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.4301 | 589 | 24.99 |
| 1.4275 | 589 | 34.99 |
| 1.4192 | 589 | 54.99 |
| 1.42235 | 589 | 49.99 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.10844 | 24.99 |
| 1.099 | 39.99 |
| 1.1016 | 34.99 |
| 1.1058 | 29.99 |
| 1.08484 | 59.99 |
| 1.08842 | 54.99 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the adsorbed molecule | -130.16 | Pd(111) | |
| Adsorption | water | 24.84 | ruthenium |
| Further physical properties of the adsorbed molecule | 36.85 – 76.85 | NaX (faujasite type zeolite) | |
| Adsorption | [Cu3(μ-OH)2(μ-Cl)2(μ-2-pymo)(μ-4,4′-bpy)3]n(SO4/2)n | ||
| Further physical properties of the adsorbed molecule | TiO2(110) | ||
| Desorption isotherm(s) | -173.16 – 126.84 | Cu(100) | |
| Further physical properties of the adsorbed molecule | 80 | 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | water-d2, [(2)H6]acetone | -10 |
| Chemical shifts, Spectrum | 13C | 25 | |
| Chemical shifts | 1H | [D3]acetonitrile | 24.84 |
| Spectrum | 1H | 20 | |
| Chemical shifts | 13C | formic acid, water | 24 |
| Spectrum | 1H | dimethylsulfoxide-d6 | 26.85 |
| Spin-spin coupling constants | CDCl3 | ||
| NMR | |||
| INDOR |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | potassium bromide | |
| Spectrum | methanol | |
| Bands | KBr | |
| Spectrum | CCl4 | 24.85 |
| Spectrum | KBr | 25-200 |
| IR |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| EI (Electron impact), Spectrum | |
| spectrum | collisional activation |
| fragmentation pattern, spectrum | collisional activation |
| spectrum, chemical ionization (CI) | collisional activation |
| collisional activation, metastable ions |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Absorption maxima | 248 | ||
| UV/VIS | |||
| Spectrum | 230 – 320 nm, Osmium(VIII)-oxid-Addukt. | ||
| Spectrum | 300 – 750 nm, in alkalischer Loesung. | ||
| Spectrum | aq. NaOH | ||
| Spectrum | Zeigt im UV unterhalb 207 nm (Dampf) kontinuierliche Absorption. |
| Description (ESR Spectroscopy) |
| Spectrum |
| ENDOR (electron-nuclear double resonance) |
| ESR |
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Raman | ||
| Spectrum | neat liquid | low frequency |
| Bands | neat (no solvent) | |
| Spectrum | H2O | in the presence of salts |
| Bands | H2O | in the presence of salts |
| Spectrum | Vergleich der Ramanspektrum von festem und fluessigem Glycol |
Route of Synthesis (ROS)
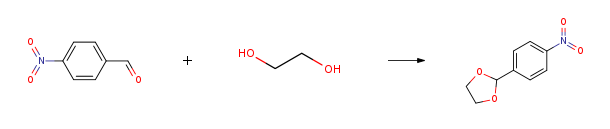
| Conditions | Yield |
| With toluene-4-sulfonic acid In toluene at 143℃; for 2h; Dean-Stark; | 100% |
| With cyclohexane at 105℃; for 1h; Dean-Stark; | 100% |
| With p-toluenesulfonic acid monohydrate In toluene for 4h; Reflux; | 100% |
| With toluene-4-sulfonic acid In toluene for 3h; Reflux; | 99% |
| With poly(ethylene glycol) 1000 based dicationic acidic ionic liquid In toluene at 80℃; for 1h; Reagent/catalyst; Time; Ionic liquid; | 99% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P270, P301+P312, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 9; Packaging Group: III; UN Number: 3082 |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 62.0684 |
| logP | -1.208 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 5 | NOAEC | 1E-05 | M | ||
| 5 | NOAEC | 1E-05 | M | ||
| 5 | NOAEC | 1E-05 | M | Cytotoxic | |
| 5 | NOAEC | 1E-05 | M | Cytotoxic | |
| 3.66 | CC50 (cytotoxic concentration) | = | 221 | µM | |
| 3.41 | CC50 (cytotoxic concentration) | = | 385 | µM | |
| 1.71 | NOAEC | 1200 | mg/L | Phytotoxic |
| Quantitative Results | ||
| 1 of 10 | Assay Description | Octanol-water distribution coefficient of the compound at pH 7.4 was determined |
| Measurement | Octanol-water distribution coefficient | |
| 2 of 10 | Biological material | rat brain |
| Assay Description | Observed Permeability-surface area in rat brain upon perfusion with compound | |
| Measurement | Observed Permeability-surface area | |
| 3 of 10 | Assay Description | Octanol-water partition coefficient of the compound was determined |
| Measurement | Octanol-water partition coefficient | |
| 4 of 10 | Assay Description | Intrinsic permeability coefficient of compound measured by PAMPA (parallel artificial membrane permeability assay) |
| Measurement | Intrinsic permeability coefficient | |
| 5 of 10 | Target | Lactaldehyde Dehydrogenase [Escherichia coli]:Wild |
| Biological material | Escherichia coli JA 102 | |
| Assay Description | Specific activity of the compound (carbon source) against lactaldehyde dehydrogenase in Escherichia coli JA-102 strain using 0.05 mM L-lactaldehyde upon incubation for at 25 degree C in 100 mM sodium glycine buffer, pH 10.5 | |
| Results | Activity not calculated | |
| 6 of 10 | Target | acetoin dehydrogenase:Wild |
| Assay Description | Maximum velocity towards D-1-amino-2-propanol oxidoreductase activity using 5 mM NAD+ cosubstrate upon incubation in Tris.HCl buffer, pH 8.4 for 5 min at 37 degree C with compound (substrate) was measured by formation of NADH | |
| Results | Vmax not calculated | |
| Measurement | Vmax | |
| 7 of 10 | Target | Glycolaldehyde Dehydrogenase [Escherichia coli]:Wild |
| Biological material | Escherichia coli JA 102 | |
| Assay Description | Specific activity of the compound against (carbon source) glycolaldehyde dehydrogenase in Escherichia coli JA-102 strain using 1 mM glycolaldehyde upon incubation for at 25 degree C in 100 mM sodium glycine buffer, pH 9.5 | |
| Results | Activity not calculated | |
| 8 of 10 | Assay Description | Lipophilicity of the compound was determined |
| Measurement | Lipophilicity | |
| 9 of 10 | Assay Description | Permeability-surface area product of the compound was determined |
| Results | log PS not calculated | |
| Measurement | log PS | |
| 10 of 10 | Assay Description | Partition coefficient of compound was evaluated |
| Measurement | Partition coefficient |
| Use Pattern |
| Ethylene glycol CAS# 107-21-1 is a widely used basic material. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
