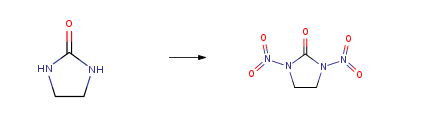
Ethyleneurea CAS#: 120-93-4; ChemWhat Code: 29264
Identification
| Product Name | Ethyleneurea |
| IUPAC Name | imidazolidin-2-one |
| Molecular Structure | 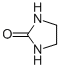 |
| CAS Registry Number | 120-93-4 |
| EINECS Number | 204-436-4 |
| MDL Number | MFCD00005257 |
| Beilstein Registry Number | 106252 |
| Synonyms | imidazolidone, 2-imidazolidone, ethyleneurea;CAS#: 120-93-4 |
| Molecular Formula | C3H6N2O |
| Molecular Weight | 86.09 |
| InChI | InChI=1S/C3H6N2O/c6-3-4-1-2-5-3/h1-2H2,(H2,4,5,6) |
| InChI Key | YAMHXTCMCPHKLN-UHFFFAOYSA-N |
| Canonical SMILES | C1CNC(=O)N1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/30193 | TWO-STEP PROCESS FOR CONVERTING CYCLIC ALKYLENE UREAS INTO THEIR CORRESPONDING ALKYLENE AMINES | 2019 |
| WO2019/30197 | PROCESS TO PREPARE PROPYLENE AMINES AND PROPYLENE AMINE DERIVATIVES | 2019 |
| EP2548870 | Process for the Synthesis of Cyclic Alkylene Ureas | 2013 |
| WO2013/87578 | DISUBSTITUTED BENZOTHIENYL-PYRROLOTRIAZINES AND THEIR USE AS FGFR KINASE INHIBITORS | 2013 |
| WO2006/111346 | SUBSTITUTED CYCLIC UREA DERIVATIVES AND THE USE THEREOF AS VANILLOID RECEPTOR 1 MODULATORS | 2006 |
Physical Data
| Appearance | White crystalline powder |
| Solubility | soluble in water |
| Refractive index | 1.5110 (estimate) |
| Melting Point, °C | Solvent (Melting Point) | Comment (Melting Point) |
| 130 – 132 | ||
| 132 | ethanol | |
| 120 – 123 | Decomposition | |
| 131 – 132 | benzene | |
| 132 – 132.5 | dioxane | |
| H2O | with:0.5 Mol.H2O (solvent).gehen bei Raumtemperatur langsam in die wasserfreien Kristalle ueber. | |
| 131 | CHCl3 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 258 – 265 | |
| 80 – 100 | 0.05 |
| 134 – 140 | 15 |
| 192 | 16 |
| 187 | 10 |
| 163 | 3 |
| Sublimation, °C | Pressure (Sublimation), Torr | Comment (Sublimation) |
| bei 120-150grad/0,1 mm | ||
| 130~140 | 0.9 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | acetonitrile | 5-(2,5,8,11-tetraoxatridecan-13-yloxy)-N1,N3-di(1,10-phenanthrolin-2-yl)isophthalamide | |
| Stability constant of the complex with … | CDCl3 | 26.85 | N,N’,N”-Tris(7-methyl-1,8-naphthyridin-2-yl)benzene-1,3,5-tricarbonamide |
| NMR spectrum of the complex | CDCl3 | 19.85 | C78H102O12, 5,11,17,23,29,35-hexa-tert-butyl-37,39,41-trimethoxy-38,40,42-tris(2-aminoethoxy)calix[6]arene |
| NMR spectrum of the complex | benzene | 1,3-bis<<(6-cholesteryloxyforamido-2-pyridyl)amino>carbonyl>benzene | |
| NMR spectrum of the complex | methanol | 1,3-bis<<(6-cholesteryloxyforamido-2-pyridyl)amino>carbonyl>benzene | |
| Association with compound | 1,2-dichloro-ethane | 1,3-bis<<(6-cholesteryloxyforamido-2-pyridyl)amino>carbonyl>benzene | |
| Association with compound | CDCl3 | 26.85 | 5-(N-phenylureido)methyl-25,26-27,28-bis(crown-3)-calix[4]arene |
| IR spectrum of the complex | KBr | 3,4,5,6-tetrahydro-3,3,6,6-tetramethylbis(pyrido<3,2-g>indolo)<2,3-a:3′,2′-j>acridine | |
| Further physical properties of the complex | CHCl3, cyclohexane | N,N’-bis<6-<4-(1-pyrenyl)butanamido>-2-pyridyl>isophthalamide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Comment (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | [(2)H6]acetone | ||
| Chemical shifts, Spectrum | 13C | [(2)H6]acetone | ||
| Chemical shifts, Spectrum | 13C | water-d2 | ||
| Chemical shifts, Spectrum | 1H | water-d2 | 19.84 | |
| Chemical shifts | 1H | aq. phosphate buffer | ||
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 37 | pH dependence |
| Chemical shifts | 15N | pyridine | 32~34 | |
| Chemical shifts | 15N | H2O | 32~34 | |
| Spectrum | 1H | various solvent(s) | 25 | |
| Spectrum | 13C | D2O | 25 | |
| Spin-spin coupling constants | dimethylsulfoxide | 32 – 34 | 1H-15N. | |
| NMR |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | paraffin | |
| Bands | KBr | 1704 – 1510 cm**(-1) |
| Spectrum | CH2Cl2 | 1770 – 1640 cm**(-1) |
| Spectrum | KBr | 10000 – 667 cm**(-1) |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| electrospray ionisation (ESI), spectrum |
| GCMS (Gas chromatography mass spectrometry), Spectrum |
| spectrum |
| electron impact (EI) |
| Description (UV/VIS Spectroscopy) |
| UV/VIS |
Route of Synthesis (ROS)
| Conditions | Yield |
| With dinitrogen pentoxide In carbon dioxide at 0 – 5℃; under 45004.5 – 60006 Torr; for 1h; liquid CO2; Experimental Procedure General procedure: General nitration procedure.12-14 A steel autoclave (25 cm3) equippedwith sapphire windows containing urethane 1c or amide 3 or 5 (10.0 mmol)was filled with liquid CO2 to 60 bar pressure and cooled to 0 °C. ThenN2O5 (2.4 g, 22.0 mmol) solution in liquid CO2 (~ 4 g) cooled to 0-5 °Cwas gradually pressed out from an auxiliary high-pressure cell by a freshCO2 flow (2 g min-1) to the reaction autoclave. During the addition, thepressure in the latter raised up to 80 bar. The reaction mixture was stirredat 0-5 °C for the time specified in Table 1. Then, CO2 was removed bydecompression and the residue was poured onto ice water (50 ml). Theresulted suspension was extracted with EtOAc (4 × 20 ml), the combinedorganic extracts were washed successively with saturated aqueous NaHCO3(2 × 20 ml) and water (25 ml) and dried over anhydrous Na2SO4. Thesolvent was removed under reduced pressure to afford corresponding nitrocompounds 2, 4 (see Table 1). Compounds 2a,b and 7 were synthesizedby similar procedures using 1.2 g (11.0 mmol) or 6.0 g (55 mmol) ofN2O5, respectively. | 93% |
| With ammonium nitrate; trifluoroacetic anhydride In nitromethane Ambient temperature; | 41% |
| With nitronium trifluoromethanesulfonate In dichloromethane for 1h; Ambient temperature; | 32.6% |
| With chloroform; dinitrogen pentoxide |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H361 (13.88%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H373 (19%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P260, P264, P280, P281, P305+P351+P338, P308+P313, P314, P337+P313, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293399 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 13/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 86.0934 |
| logP | -0.86 |
| HBA | 3 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 41.13 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Parameter | Value (qual) | Value (quant) | Unit | Target |
| percentage(Cell growth effect) | = | 108 | % | |
| percentage(Cell growth effect) | = | 88 | % | |
| Activity(Erythroid maturation) | = | 4 | % | |
| inhibition rate | = | 60.07 | % | heparanase:Wild |
| inhibition rate | = | 99.95 | % | heparanase:Wild |
| Quantitative Results | ||
| 1 of 4 | Assay Description | Log P value of the compound was evaluated |
| 2 of 4 | Results | DL 50: 300 mg/kg (i.p., mouse) |
| 3 of 4 | Assay Description | Effect : metabolic Target : rabbit liver cytosol Bioassay : IMI: imidacloprid effect on <3H>-IMI metabolism determined; incubated with NADPH in argon atmosphere |
| Results | slight inhibition of <3H>-IMI metabolism; percentage values for conversion IMI to polar metabolites in range of 8-16 percent (control values in range of 21-24 percent) | |
| 4 of 4 | Results | in vitro no antiviral effect against poliovirus 1, Semliki Forest, fowl plague, Newcastle disease, pseudorabies, vaccinia viruses at 0.1 ml of 2percent solution |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 2.92 | LC50(Lethal dose) | = | 1.19 | mM | Death |
| LD50 | > | 4000 | mg/kg | ||
| LD50 | > | 46.5 | mM/kg |
| Use Pattern |
| Ethyleneurea CAS#: 120-93-4 Pharmaceuticals |
| Ethyleneurea CAS#: 120-93-4 altering the refractive power of a cornea |
| Ethyleneurea CAS#: 120-93-4 altering the refractive power of an isolated cornea |
| crosslinking collagen present in a collagenous tissue |
| crosslinking collagen present in cornea of a subject afflicted with keratectasia |
| inhibiting loss of structural integrity of a collagenous tissue during transplantation-related transport |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.caming.com/ |
| BASF | https://www.basf.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |