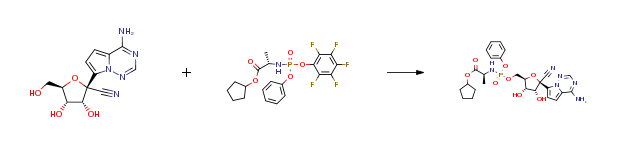
EVO984 CAS#: 1191237-69-0; ChemWhat Code: 1372756
Identification
| Product Name | EVO984 |
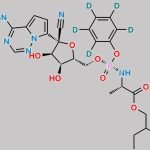
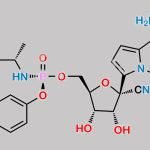
| IUPAC Name | (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)oxolane-2-carbonitrile |
| Molecular Structure | 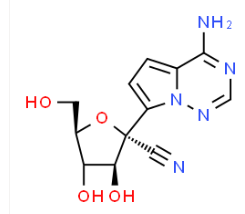 |
| CAS Registry Number | 1191237-69-0 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | (2R,3R,4S,5R)-2-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile, (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile, 1′-cyano-4-aza-7,9-dideazaadenosine, GS-441524 CAS#: 1191237-69-0 CAS 1191237-69-0 CAS No. 1191237-69-0 |
| Molecular Formula | C12H13N5O4 |
| Molecular Weight | 291.263 |
| InChI | InChI=1S/C12H13N5O4/c13-4-12(10(20)9(19)7(3-18)21-12)8-2-1-6-11(14)15-5-16-17(6)8/h1-2,5,7,9-10,18-20H,3H2,(H2,14,15,16)/t7-,9?,10+,12+/m1/s1 |
| InChI Key | BRDWIEOJOWJCLU-AKDWBFHISA-N |
| Canonical SMILES | c1cc(n2c1c(ncn2)N)[C@]3([C@H](C([C@H](O3)CO)O)O)C#N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110724174 | Pyrrolotriazinoid-based compound as, well as application thereof (by machine translation) | 2020 |
| CN110613726 | Drug for application of, nucleoside compound and preparation method thereof (by machine translation) | 2019 |
| US2017/71964 | METHODS FOR TREATING ARENAVIRIDAE AND CORONAVIRIDAE VIRUS INFECTIONS | 2017 |
| WO2016/69826 | METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS | 2016 |
| WO2012/12776 | METHODS AND COMPOUNDS FOR TREATING PARAMYXOVIRIDAE VIRUS INFECTIONS | 2012 |
Physical Data
| Appearance | No data available |
| Solubility | No data available |
| Density | 1.84±0.1 g/cm3(Predicted) |
| Acidity coefficient (pKa) | 12.13±0.70(Predicted) |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.84±0.1 g/cm3 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 | 1H NMR (400 MHz, DMSO) δ 7.91 (s, 1H), 7.80-8.00 (br s, 2H), 6.85-6.89 (m, 2H), 6.07 (d, J=6.0 Hz, 1H), 5.17 (br s, 1H), 4.90 (br s, 1H), 4.63 (t, J=3.9 Hz, 1H), 4.02-4.06 (m, 1H), 3.94 (br s, 1H), 3.48-3.64 (m, 2H). |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | NMR (400 MHZ, DMSO), 156.0, 148.3, 124.3, 117.8, 117.0, 111.2, 101.3, 85.8, 79.0, 74.7, 70.5, 61.4. | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 | NMR (400 MHz, DMSO) δ 7.91 (s, 1H), 7.80-8.00 (br s, 2H), 6.85-6.89 (m, 2H), 6.07 (d, J= 6.0 Hz, 1H), 5.17 (br s, 1H), 4.90 (br s, 1H), 4.63 (t, J= 3.9 Hz, 1H), 4.02-4.06 (m, 1H), 3.94 (br s, 1H), 3.48- 3.64 (m, 2H). |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 13C NMR (400 MHZ, DMSO), 156.0, 148.3, 124.3, 117.8, 117.0, 111.2, 101.3, 85.8, 79.0, 74.7, 70.5, 61.4. | |
| Chemical shifts | 1H | water-d2 | 300 | 1H NMR (300 MHz, D2O) δ 7.96 (s, 1H), 7.20 (d, J= 4.8 Hz, 1H), 6.91 (d, J= 4.8 Hz, 1H), 4.97 (d, J= 4.4 Hz, 1H), 4.56-4.62 (m, 1H), 4.08-4.14 (m, 1H), 3.90 (dd, J = 12.9, 2.4 Hz, 1H), 3.70 (dd, J = 13.2, 4.5 Hz, 1H). |
| 1H | dimethylsulfoxide-d6 | 400 | 1H NMR (400 MHz, DMSO) δ 7.91 (s, 1H), 7.80-8.00 (br s, 2H), 6.85-6.89 (m, 2H), 6.07 (d, J= 6.0 Hz, 1H), 5.17 (br s, 1H), 4.90 (br s, 1H), 4.63 (t, J= 3.9 Hz, 1H), 4.02-4.06 (m, 1H), 3.94 (br s, 1H), 3.48-3.64 (m, 2H). | |
| 13C | dimethylsulfoxide-d6 | 13C NMR (400 MHZ, DMSO), 156.0, 148.3, 124.3, 117.8, 1 17.0, 111.2, 101.3, 85.8, 79.0, 74.7, 70.5, 61.4 |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), spectrum |
| Spectrum |
| liquid chromatography mass spectrometry (LCMS), spectrum |
| LCMS (Liquid chromatography mass spectrometry) |
Route of Synthesis (ROS)
| Conditions | Yield |
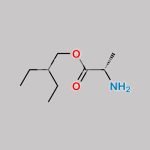
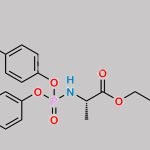
| With tert-butylmagnesium chloride In tetrahydrofuran at 0 – 40℃; for 2h; Experimental Procedure Preparation process: 1.50 g of a nucleoside compound of the formula (I-1) is added to a round bottom flask,200 mL of tetrahydrofuran and 2.47 g of active phosphate of formula (F), cooled to 0 ° C in an ice bath,Slowly add 10 mL of tert-butyl magnesium chloride solution (1.0M tetrahydrofuran solution) under stirring.After completion of the dropwise addition, the temperature was raised to 40 ° C and the reaction was carried out for 2 hours. After the reaction, the reaction solution was concentrated under reduced pressure,The concentrate was added to 200 mL of dichloromethane and washed twice with 50 mL of a saturated sodium chloride solution.The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure.The concentrated residue was separated by silica gel column chromatography using a mixture of ethyl acetate / petroleum ether = 1/1 as an eluent.1.58 g of the compound of the formula (I-6) was obtained, and the calculated yield was 52%. | 52% |
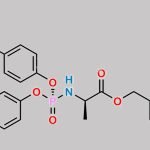
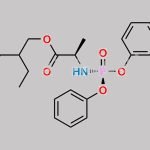
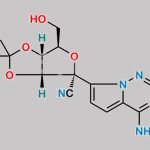
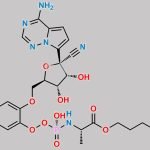
| Stage #1: (2R,3R,4S,5R)-2-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile With tert-butylmagnesium chloride In tetrahydrofuran at 20℃; for 0.5h; Cooling with ice; Stage #2: cyclopentyl ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alaninate In tetrahydrofuran at 20℃; for 24h; | 23% |
| Stage #1: (2R,3R,4S,5R)-2-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile With tert-butylmagnesium chloride In tetrahydrofuran at 20℃; for 0.5h; Cooling with ice; Stage #2: cyclopentyl ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alaninate In tetrahydrofuran at 20℃; for 24.05h; Experimental Procedure Compound 1 (100 mg, 0.34 mmol) was dissolved in THF (2 mL) and cooled under ice water bath. Then 1M t-BuMgCl (0.52 mL, 0.77 mmol) was added dropwise slowly. The resulting mixture was stirred for 30 min at room temperature. Then compound K (Prepared according to WO2012075140, 247 mg, 0.52 mmol) in THF (2 mL) was added over 5 min and the resulting mixture was stirred for 24 h at room temperature, diluted with EtOAc, cooled under ice-water bath, treated with aq NaHCO3 (2 mL), washed with brine, dried with sodium sulfate, and concentrated in vacuo. The resulting mixture was purified by silica gel column chromatography ( MeOH 0 to 20% in DCM) and prep-HPLC (acetonitrile 10 to 80% in water) to give example 28 (47 mg, 23% as a 27:1 mixture of diastereomers). 1H NMR (400 MHz, CD3OD) δ 7.85 (s, 1H), 7.33-7.22 (m, 2H), 7.14 (tdd, J=7.6, 2.1, 1.1 Hz, 3H), 6.95-6.87 (m, 2H), 5.13-5.00 (m, 1H), 4.78 (d, J=5.4 Hz, 1H), 4.48-4.35 (m, 2H), 4.30 (ddd, J=10.6, 5.7, 3.6 Hz, 1H), 4.19 (t, J=5.4 Hz, 1H), 3.78 (dq, J=9.2, 7.1 Hz, 1H), 1.81 (dtd, J=12.5, 5.9, 2.4 Hz, 2H), 1.74-1.49 (m, 6H), 1.21 (dd, J=7.1, 1.2 Hz, 3H). MS m/z=587 (M-1)+ | 29% |
| Stage #1: (2R,3R,4S,5R)-2-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile With tert-butylmagnesium chloride In tetrahydrofuran at 20℃; for 0.5h; Cooling with ice; Stage #2: cyclopentyl ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alaninate In tetrahydrofuran at 20℃; for 24h; |
Safety and Hazards
| Pictogram(s) | No data available |
| Signal | No data available |
| GHS Hazard Statements | No data available [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | No data available (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 291.266 |
| logP | -1.616 |
| HBA | 9 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 149.92 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 1 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Cell | Dose | Effect |
| 7.28 | inhibition rate | 100 | CRFK cell line | 1 μM | Anticytopathic | ||
| 6.74 | EC50(viral titre reduction) | 0.18 | μM | airway epithelium cell | antiviral agent | ||
| 6.66 | IC50 | 0.22 | µM | HAEC cell line | antiviral agent | ||
| 6.32 | EC50(Virus-induced cytopathic effect) | = | 0.48 | µM | HEp-2 cell line | antiviral agent | |
| 6 | IC50 | 1000 | nM | HeLa cell line | antifungal agent | ||
| 5.77 | EC50(Virus replication) | = | 1.71 | μM | Vero cell line | antiviral agent | |
| 5.28 | IC90 | > | 47 | μM | Vero cell line | 0.047 – 47 μM | Anticytopathic |
| 4.55 | EC50(Virus replication) | = | 27.9 | μM | MDCK cell line | antiviral agent | |
| 1 | CC50 (cytotoxic concentration)(Cell growth) | > | 89 | μM | Huh-7 cell line | Cytotoxic |
| In vitro: Animal Model |
| Quantitative Results |
| Parameter | Value (qual) | Biological Species | Animal Model | Dose | Dosing regimen | Effect |
| lymphocyte count increase | Active | cat | experimental peritonitis | 2 – 5 mg/kg | Repeated | antiviral agent |
| body temperature decrease | Active | cat | experimental peritonitis | 2 – 5 mg/kg | Repeated | antiviral agent |
| Metabolism |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Cell | Dose |
| 6 | concentration (parameter) | 1 | μM | CRFK cell line | 1μM |
| Pharmacokinetic |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Dose |
| 6.1 | concentration (parameter) | 0.8 – 2.8 | μM | 10 mg/kg |
| 5.62 | concentration (parameter) | 2.4 – 4.3 | μM | 10 mg/kg |
| 5.1 | concentration (parameter) | 8 – 20 | μM | 5 mg/kg |
| 4.96 | concentration (parameter) | 11 – 12.8 | μM | 5 mg/kg |
| AUC | 38.9 | μmol*h/l | 10 mg/kg | |
| AUC | 43.8 | μmol*h/l | 5 mg/kg | |
| concentration (parameter) | 5 mg/kg | |||
| concentration (parameter) | 5 mg/kg |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Cell | Effect |
| 1 | CC50 (cytotoxic concentration)(Cell growth) | > | 30 | μM | Vero C1008 cell line | Cytotoxic |
| 1 | CC50 (cytotoxic concentration)(Cell growth) | > | 30 | μM | MRC-5 cell line | Cytotoxic |
| 1 | CC50 (cytotoxic concentration) | > | 57 | μM | MT-4 cell line | Cytotoxic |
| 1 | CC50 (cytotoxic concentration) | > | 88 | μM | Huh-7-HCV genotype 1b (Con 1) replicon cell line | Cytotoxic |
| 1 | inhibition rate | > | 100 | μM | HEp-2 cell line | Cytotoxic |
| 1 | inhibition rate | > | 100 | μM | Calu-3 cell line | Cytotoxic |
| 1 | CC50 (cytotoxic concentration) | > | 300 | μM | SR-CDF1 DBT cell line | Cytotoxic |
| 1 | IC50 | > | 1060 | μM | CRFK cell line | Cytotoxic |
| 1 | toxicity rate | Not active |
| Use Pattern |
| EVO984 CAS#: 1191237-69-0 is also as Pharmaceuticals |
| EVO984 CAS#: 1191237-69-0 use to cure feline Coronavirus infections |
| feline enteric coronavirus (FECV) |
| feline infectious peritonitis virus (FIPV) |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |