FERRIC CITRATE CAS#: 2338-05-8; ChemWhat Code: 37472
Identification
| Product Name | FERRIC CITRATE |
| IUPAC Name | 2-hydroxypropane-1,2,3-tricarboxylic acid;iron |
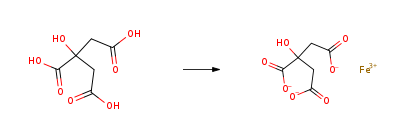
| Molecular Structure |  |
| CAS Registry Number | 2338-05-8 |
| EINECS Number | 219-045-4 |
| MDL Number | MFCD00013098 |
| Beilstein Registry Number | 4052503 |
| Synonyms | ferric citrate, iron(III) citrate, Fe(III) citrate, iron citrate, Fe(3+) citrate, citric acid, KRX-0502 CAS#: 2338-05-8 CAS No.: 2338-05-8 |
| Molecular Formula | C6H13FeO11 |
| Molecular Weight | 317.006 |
| InChI | InChI=1S/C5H6N2/c6-5-2-1-3-7-4-5/h1-4H,6H2 |
| InChI Key | VTIIZUHZMUVVCB-UHFFFAOYSA-K |
| Canonical SMILES | C(C(=O)[O-])C(CC(=O)[O-])(C(=O)[O-])O.O.O.O.O.[Fe+3] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2012/121703 | TABLET CONTAINING FERRIC CITRATE | 2012 |
| WO2009/52924 | PROCESS FOR PRODUCING CARNOSOL FROM CARNOSIC ACID | 2009 |
| US6352706 | Naturally occurring enhancer of metal toxicants in molluscs | 2002 |
Physical Data
| Appearance | Red-brown flake |
| Solubility | Soluble in water |
| Sensitivity | 0: forms stable aqueous solutions |
| Comment (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| sorption diagram | activated carbon |
| Description (Electrochemical Characteristics) | Solvent (Electrochemical Characteristics) | pH-Value (Electrochemical Characteristics) | Comment (Electrochemical Characteristics) |
| cyclovoltammetry | water | 6 | saturated calomel electrode (SCE); potential diagram; 1 M sodium acetate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | water-d2 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | |
| Spectrum | water | 1100 cm**-1 – 1800 cm**-1 |
| Bands | water | 1100 cm**-1 – 1800 cm**-1 |
| Bands | potassium bromide | 400 cm**-1 – 4000 cm**-1 |
| Bands | KBr | 400 cm**-1 – 4000 cm**-1 |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| LCMS (Liquid chromatography mass spectrometry) | Molecular peak |
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: citric acid With sodium hydroxide In water for 0.5h; Stage #2: With iron(III) chloride hexahydrate In water at 50 – 55℃; for 1h; Experimental Procedure 2 Example-2: Preparation of Ferric Citrate Example-2: Preparation of Ferric Citrate To a 2.0 L four-neck round bottom flask, 200 ml purified water and 44.4 gm sodium hydroxide were added and stirred to make a solution. To the reaction mass, 77.7 gm citric acid monohydrate was added and stirred for 30 minutes. Thereafter, 100 gm ferric chloride hexahydrate was added to the reaction mass and further stirred fori hour at 50-55°C. The reaction mass was cooled to 25 °C and subsequently, 1200 ml of methanol was slowly added to the reaction mass and stirred for 1 hour. Precipitated product was filtered and washed with 100 ml of methanol. The wet product was dried under reduced pressure. Yield: 85% molar yield Description: dark brown colored to red colored powder | 85% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P305+P351+P338, and P337+P313 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 291814 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 9/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 244.948 |
| logP | -3.69 |
| HBA | 7 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 140.62 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 1 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Cell | Dose | Effect |
| 4.3 | inhibition rate | Active | A-549 cell line | 50 μM | antiviral agent |
| 4 | inhibition rate | Active | RD cell line | 100 μM | antiviral agent |
| Quantitative Results | ||
| 1 of 7 | Assay Description | Effect : Body Weight Gain; increase of Target : SD rat Bioassay : Effects on Body Weight Gain and Food Intake Table 11 shows the individual body weight gain and food intake of the rats in the groups which were fed iron-containing food 1 (ferric citrate), food 2 (heme iron) and food 3 (ferrichrysin) after the development of iron deficiency anemia, as well as the rats |
| Results | The weight gains of the groups which consumed test compound increased to 79.8 (g/3 weeks) as compared to those of the control group (65.3 g/3 weeks) | |
| 2 of 7 | Assay Description | Effect : food intake; increase of Target : SD rat Bioassay : Effects on Body Weight Gain and Food Intake Table 11 shows the individual body weight gain and food intake of the rats in the groups which were fed iron-containing food 1 (ferric citrate), food 2 (heme iron) and food 3 (ferrichrysin) after the development of iron deficiency anemia, as well as the rats |
| Results | The food intakes of the groups which consumed test compound increased to 384.8 (g/3 weeks) as compared to those of the control group (336.2 g/3 weeks) | |
| 3 of 7 | Assay Description | Effect : Iron Concentration in the Liver; effect on Target : SD rat Bioassay : Measurement of Iron Concentration in the Liver After the induction of iron deficiency anemia, rats were fed iron-containing food 1 (ferric citrate), food 2 (heme iron) and food 3 (ferrichrysin),respectively, for three weeks. Rats of the control group were fed food 1 (ferric citrate) from the beginning, |
| Results | The groups which were fed the test compound had developed severe iron deficiency anemia, and accordingly, the amounts of iron stored in the livers of these groups were decreased to 21.6 ppm as compared to the control (63.3 ppm) group | |
| 4 of 7 | Assay Description | Effect : Liver and Kidney Functions; effect on Target : SD rat Bioassay : Effects on Liver and Kidney Functions Measurements were made by a usual method of the concentrations of protein, ALT (alanine aminotransferase), AST (aspartate aminotransferase) and creatinine in the serums of the groups of rats which were fed iron-containing food 1 (ferric citrate), food 2 (heme iron) |
| Results | The serum analysis values for the test group were within normal ranges, and there were no results indicating abnormality in the liver or kidney function. | |
| 5 of 7 | Assay Description | Effect : serum iron concentration; increase of Target : SD rat Bioassay : Measurement of Serum Iron Concentration Serum was prepared from the blood wholly exsanguinated from the rats of each group after the rats had been given their iron-containing food, so as to measure iron concentrations in the serums by a usual method. The results are shown in Table 8 below. Table 8 |
| Results | the serum iron concentrations of the groups which consumed the foods containing test compound increased to 131 μg/dl (control: 106.2 μg/dl) | |
| 6 of 7 | Target | Hemoglobin:Wild |
| Assay Description | Effect : blood hemoglobin concentration; increase of Bioassay : Measurement of Hemoglobin Concentration in the Blood Blood was collected from the groups of rats which were fed iron-containing food 1 (ferric citrate), food 2 (heme iron) and food 3 (ferrichrysin) after the development of iron deficiency anemia both prior to and 3 weeks after giving these foods, so | |
| Results | The hemoglobin values of the groups which consumed test compound increased from 5.7 g/dl to 13.3 g/dl (control: 14.2-14.8 g/dl) | |
| 7 of 10 | Biological material | human |
| Assay Description | Effect : serum creatinine level; effect on Bioassay : Decrease in Serum Creatinine Level[0173] Glomerular filtration rate (GFR) level correlates with structural kidney damage and is used as a golden standard to measure kidney function. GFR can be estimated by the biomarkers serum creatinine. As renal function deteriorates, kidney lost its function to excrete | |
| Results | at 6 g/day title compound, serum creatinine was 0-9.6 mg/dL; figures are given |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Cell | Does | Effect |
| 1 | inhibition rate | Not active | Caco-2 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| 1 | inhibition rate | Not active | HT-29 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| 1 | inhibition rate | Not active | HuTu 80 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| 1 | inhibition rate | Not active | Caco-2 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| 1 | inhibition rate | Not active | HT-29 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| 1 | inhibition rate | Not active | HuTu 80 cell line | 0.015 – 0.89 mM | antineoplastic agent |
| inhibition rate(Cell growth) | Active | Burkitt lymphoma cell line | antineoplastic agent |
| Use Pattern |
| FERRIC CITRATE CAS#: 2338-05-8 is as Pharmaceuticals |
| preparation of pharmaceutical composition for inhibiting flaviviridae virus |
| FERRIC CITRATE CAS#: 2338-05-8 is as hyperphosphatemia |
| FERRIC CITRATE CAS#: 2338-05-8 is as metabolic acidosis |
| Agricultural use |
| in combination with ethanolamine, gallnut extract, garlic extract, methyl cellulose calcium, polycarbonate resin, polyacrylamide, 6-furfurylaminopurine, brown sugar solution, sodium alginate, potassium peroxide, magnesium carbonate and slow release material |
| rooting agent composition to increase growth vigor of plant roots |
| decreased left ventricular (LV) ejection fraction |
| heart failure |
| reducing mortality and morbidity related to adverse cardiac events in subjects with chronic kidney disease |
| chronic kidney disease (CKD) |
| end stage renal disease (ESRD) |
| improving at least one iron storage parameter by oral administration to chronic kidney disease (CKD) patient |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |