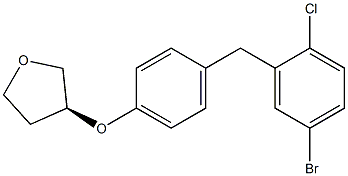
Stage #1: (S)-4-bromo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)benzene With n-butyllithium In tetrahydrofuran; hexane; toluene at -78℃; for 1h; Inert atmosphere; Large scale;
Stage #2: (3R,4S,5R,6R)-3,4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one In tetrahydrofuran; hexane; toluene at -78℃; for 3h; Large scale;
Stage #3: methanol With methanesulfonic acid In tetrahydrofuran; hexane; toluene at 0 – 40℃; for 10h; Large scale;
Experimental Procedure
7 Example 7 preparation of 1-Chloro-4-(1-methoxy-D-glucopyranose-1-yl)2-(4-(S)-tetrahydrofuran-3-yloxy-benzyl)-benzene (VII)
Take (S)-3-(4-(5-bromo-2-chlorobenzyl)phenoxy) tetrahydrofuran 183kg,400kg anhydrous THF/toluene (1:4) was added to the nitrogen-dried 3000 litre reactor.The liquid nitrogen was cooled to -78°, and hexane solution of 1. 6 mol·L-1 n-butyllithium was slowly added dropwise.Stirring was continued at this temperature for 1 h; 600 kg of a 2,3,4,6-tetra-O-trimethylsilyl-D-glucono-lactone (256 kg) in toluene solution cooled to -78°C was slowly added dropwise to the above 600 kg. In the reaction solution,-78° reaction for 3 h, after the basic reaction of TLC detection is completed,At this temperature is added 500kg of methanesulfonic acid in methanol solution (methanesulfonic acid 225kg + methanol 275kg);The reaction was stirred at 0 ° C 4h, and then warmed to 40 ° C stirred reaction 6h;5 mol•L-1 sodium hydroxide aqueous solution was added to the reaction solution, and the pH was adjusted to 7-8; stirring for 30 min,Extract with ethyl acetate (300kg×2)The organic phase is washed with saturated aqueous sodium chloride solution until neutral, then dried over anhydrous sodium sulfate and filtered.The filtrate was concentrated to dryness to give 208 kg of a light yellow viscous oil with a yield of 87%. | 87% |
Stage #1: (S)-4-bromo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)benzene; (3R,4S,5R,6R)-3,4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one With n-butyllithium In tetrahydrofuran; hexane; toluene at -78℃; for 3h; Inert atmosphere; Large scale;
Stage #2: methanol With methanesulfonic acid In tetrahydrofuran; hexane; toluene at 0 – 40℃; for 10h; Inert atmosphere; Large scale;
Experimental Procedure
6 Example 6 1-Chloro-4-(1-methoxy-D-glucopyranose-1-yl)-2-(4-(S)-tetrahydrofuran-3-yloxy-benzyl) – Preparation of Benzene (VII)
Take (5)-3-(4-(5-bromo-2-chlorobenzyl)phenoxy) tetrahydrofuran (91 kg), anhydrous tetrahydrofuran/toluene (200 kg (1:4)), and add it to a nitrogen-dried 2000-liter reactor. , Liquid nitrogen was cooled to -78 °C, was slowly added dropwise 1. 6 mol-L” 1 n-butyllithium hexane solution 120L, maintaining the temperature stirred for 1h;A 300 kg toluene solution of 2,3,4,6-tetra-O-trimethylsilyl-D-glucono lactone (130 kg) cooled to -78C was slowly added dropwise to the above reaction solution, -78 °C reaction 3 h, TLC test after the completion of the basic reaction, at this temperature was added 300kg of methanol solution of methanesulfonic acid (methanesulfonic acid 115kg + methanol 185kg); stirred at 0 °C reaction 4.0h, and then heated to 40 °C The reaction was stirred for 6 h. After the reaction was completed, 5 mol.I/1 aqueous sodium hydroxide solution was added to the reaction solution to adjust the pH to 7 – 8; stirred for 30 min, extracted with ethyl acetate (200 kg X 2), and the organic phase was washed with a saturated aqueous solution of sodium chloride. Neutral, then dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to give 100 kg of pale yellow viscous oil in a yield of 84%. | 84% |



![Route of Synthesis (ROS) of Furan, 3-[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy]tetrahydro-, (3S)-](https://www.chemwhat.com/wp-content/uploads/2018/12/Route-of-Synthesis-ROS-of-Furan-3-4-5-broMo-2-chlorophenylMethylphenoxytetrahydro-3S--970x141.png)