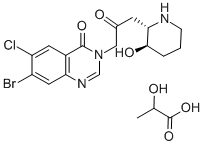
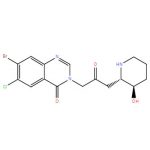
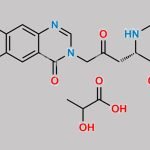
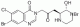Halofuginone Lactate CAS#: 82186-71-8; ChemWhat Code: 1191677
Identification
| Patent Information | ||
| No data available |
Physical Data
| Appearance | White or off-white powder |
| Melting Point | >70°C dec. |
| Boiling Point | 595.8 °C at 760 mmHg |
| Flash Point | 314.1 °C |
| Solubility | DMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming) |
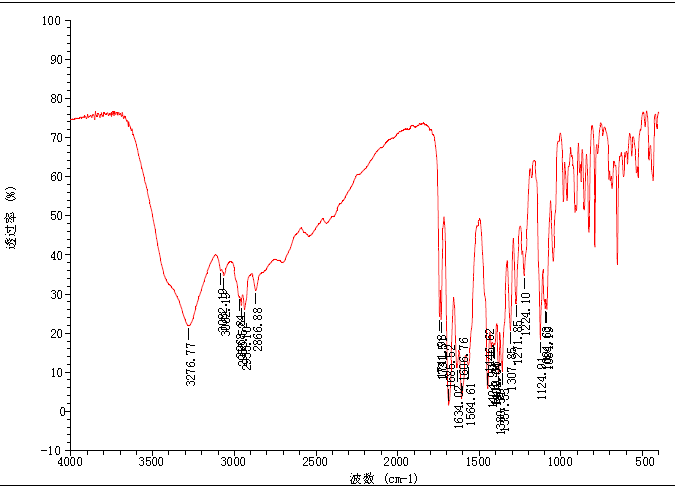
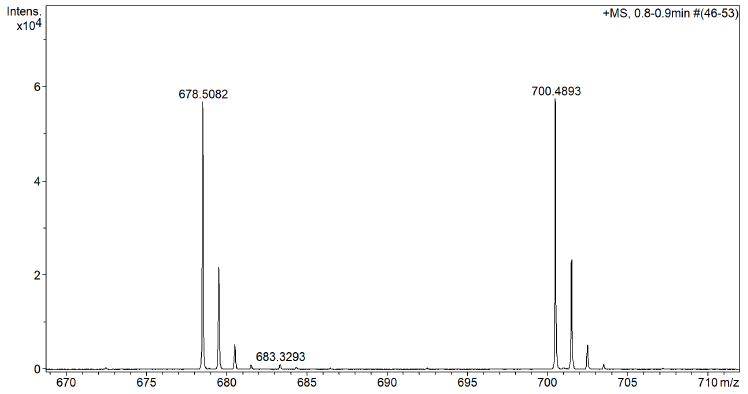
Spectra
Route of Synthesis (ROS)
| No data available |
Safety and Hazards
| Pictogram(s) |      |
| Signal | Danger |
| GHS Hazard Statements | H300: Fatal if swallowed [Danger Acute toxicity, oral] H301: Toxic if swallowed [Danger Acute toxicity, oral] H310: Fatal in contact with skin [Danger Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H330: Fatal if inhaled [Danger Acute toxicity, inhalation] H361: Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Environmentally hazardous substance, solid, n.o.s. ; Class 9; Packaging Group: III; UN Number: 3077 |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Keep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 years |
| Shelf Life | |
| Market Price | USD 2300/kg |
| Use Pattern |
| Halofuginone Lactate CAS#: 82186-71-8 is used fvor the treatment of scleroderma, cancer, and restenosis. |
| Halofuginone Lactate CAS#: 82186-71-8 is a low molecular weight quinazolinone alkaloid, and a potent inhibitor of collagen alpha1(I) and matrix metalloproteinase 2 (MMP-2) gene expression. Halofuginone also effectively suppresses tumor progression and metastasis in mice. Collgard Biopharmaceuticals is developing halofuginone for the treatment of scleroderma and received orphan drug designation from the U.S. Food and Drug Administration in March, 2000. |
| Halofuginone (RU-19110) is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. |
| Halofuginone is a potent inhibitor of collagen a1(I) and matrix metalloproteinase 2 (MMP-2) gene expression. Halofuginone also suppresses extracellular matrix deposition and cell proliferation. The profound antitumoral effect of halofuginone is attributed to its combined inhibition of the tumor stromal support, vascularization, invasiveness, and cell proliferation. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |