HATU CAS#: 148893-10-1; ChemWhat Code: 93506
Identification
| Product Name | HATU |
| IUPAC Name | [dimethylamino(triazolo[4,5-b]pyridin-3-yloxy)methylidene]-dimethylazanium;hexafluorophosphate |
| Molecular Structure | 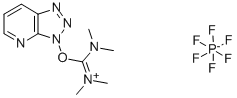 |
| CAS Registry Number | 148893-10-1 |
| NACRES | NA.22 |
| MDL Number | MFCD27957364 |
| Synonyms | HATU 148893-10-1 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate O-(7-Azabenzotriazol-1-yl)-N,N,N,N-tetramethyl uronium hexafluorophosphate O-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate |
| Molecular Formula | C10H15N6O*F6P |
| Molecular Weight | 380.233 |
| InChI | InChI=1S/C10H15N6O.F6P/c1-14(2)10(15(3)4)17-16-9-8(12-13-16)6-5-7-11-9;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1 |
| InChI Key | JNWBBCNCSMBKNE-UHFFFAOYSA-N |
| Canonical SMILES | CN(C)C(=[N+](C)C)ON1C2=C(C=CC=N2)N=N1.F[P-](F)(F)(F)(F)F |
| Patent Information | ||
| No data available |
Physical Data
| Appearance | White or almost white powder |
| Solubility | Soluble in acetonitrile. Insoluble in water |
| Melting Point, °C | Solvent (Melting Point) |
| 183-185 |
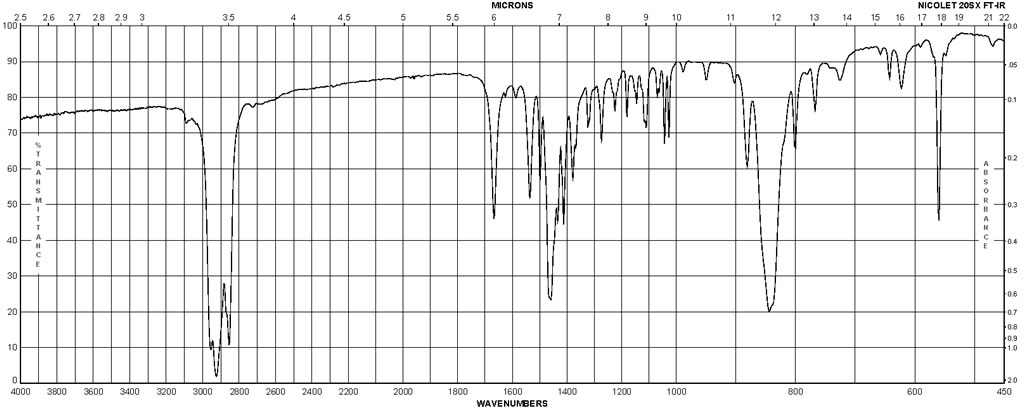
Spectra
Route of Synthesis (ROS)
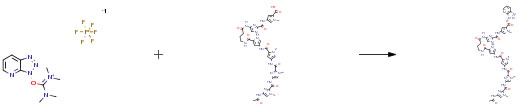
| Conditions | Yield |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 4h; Experimental Procedure Compound (3-2-1) (3.5 mg, 3.98 mmol) prepared by a solid-phase synthesis was dissolved in DMF (75 μL). Then, iPr2NEt (1.4 μL, 8.04 mmol) and HATU (1.4 mg, 3.68 mmol) were added to the resulting solution. The resulting mixture was stirred at room temperature for 4 hours in a nitrogen atmosphere. After confirmation of completion of the reaction, compound (3-2-3) (1.3 mg, 7.38 mmol) and iPr2NEt (1.3 μL, 7.46 mmol) were added thereto. The resulting mixture was stirred overnight at room temperature in a nitrogen atmosphere. After the reaction, the solvent in the reaction solution was distilled off. The residue was filtrated using a KIRIYAMA funnel and washed with CH2Cl2 and H2O. As a result, crude crystals of compound (3-2-4) were produced (3.0 mg, 73percent). 1H-NMR (500 MHz, DMSO-d6) δ: 11.33 (brs, 1H; NH), 10.34 (s, 1H; NH), 10.33 (s, 1H; NH), 10.27 (s, 1H; NH), 9.92 (s, 2H; NH), 9.73 (s, 1H; NH), 9.32 (brs, 1H; NH), 8.01 (brt, 1H; NH), 7.94 (s, 1H; CH), 7.56 (s, 1H; Im-H), 7.50 (s, 1H; Im-H), 7.46 (s, 1H; Im-H), 7.40 (brd, 1H, J = 8.5 Hz; CH), 7.31 (brd, 1H, J = 8.5 Hz; CH), 7.30 (d, 1H, J = 1.5 Hz; Py-H), 7.26 (d, 1H, J = 1.5 Hz; Py-H), 7.17 (d, 1H, J = 1.5 Hz; Py-H), 7.16 (s, 2H; y-Hx2), 6.90 (d, 1H, J = 1.5 Hz; Py-H), 6.85 (brs, 1H; CH), 4.00 (s, 3H; NCH3), 3.97 (s, 3H; NCH3), 3.95 (s, 3H; NCH3), 3.85 (s, 3H; NCH3), 3.84 (s, 3H; NCH3), 3.80 (s, 3H; NCH3), 3.20 (dt, 2H, J = 6.0 and 7.5 Hz; CH2), 2.36 (t, 2H, J = 7.5 Hz; CH2), 2.04 (s, 3H; COCH3), 1.79 (qu, 2H, J = 7.5 Hz; CH2); ESI-TOFMS m/e calcd. for C48H51N18O10 [M+ + H] 1039.40, found 1039.39 |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H228: Flammable solid [Danger Flammable solids] H315: Causes skin irritation [Warning Skin corrosion/irritation] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P210, P240, P241, P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P370+P378, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website |
Other Data
| Transportation | Class 4.1; Packaging Group: II; UN Number: 1325 |
| Under 2-8°C away from light | |
| HS Code | 293399 |
| Storage | Under 2-8°C away from light |
| Shelf Life | 1 year |
| Market Price | USD 850/kg |
| Use Pattern |
| Reagent for: Synthesis of Aurora A kinase inhibitors HPLC assay to determine D- and L- acid enantiomers in human plasma Amide bond formation reactions |
| Catalyst for: Selective acylation Selecocyclization-oxidation deselenation sequence |
| Peptide coupling reagent. Preparation of N-arylsulfonamide-linked peptides by solid-phase synthesis. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | https://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |