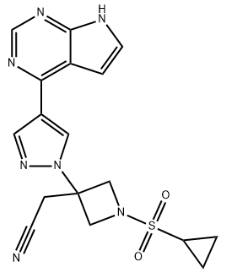
Ilunocitinib CAS#: 1187594-14-4; ChemWhat Code: 1490118
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2009/233903 | AZETIDINE AND CYCLOBUTANE DERIVATIVES AS JAK INHIBITORS | 2009 |
Physical Data
| Appearance | White powder |
Spectra
| Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| 1H | dimethylsulfoxide-d6 | 400 |
Route of Synthesis (ROS)
| Conditions | Yield |
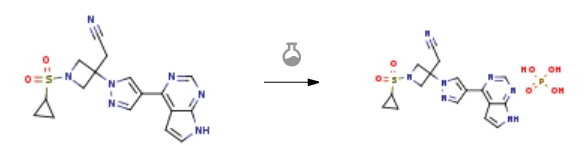
| With phosphoric acid In ethanol; acetonitrile at 20 – 67℃; Experimental Procedure 81 2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(cyclopropylsulfonyl)azetidin-3-yl)acetonitrile phosphoric acid salt To a 1 L round bottom flask equipped with a stir bar, an addition funnel, a nitrogen inlet and a condenser was charged 2-(3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(cyclopropylsulfonyl)azetidin-3-yl)acetonitrile (27, 11.3 g, 29.5 mmol) and acetonitrile (275 ML) at room temperature. The resulting mixture was warmed to 70° C. before being charged with ethanol (EtOH, 150 mL) in three portions at 70° C. The resulting homogeneous solution was filtered into a clean 1 L round bottom flask equipped with the overhead stirring, an addition funnel, a nitrogen inlet and a condenser. The mixture was then warmed to 67° C. producing a homogeneous solution again. A solution of phosphoric acid (H3PO4, 3.03 g, 30.9 mmol, 1.05 equiv) in ethanol (EtOH, 30 mL) was then charged drop wise to the solution over ten minutes at 67° C. The solution was still homogeneous after the end of the addition of phosphoric acid ethanol solution. The resulting reaction mixture was stirred at 67° C. for 10 minutes before being gradually cooled down to room temperature and stirred at room temperature for 19 h. The solids were collected by filtration and washed with acetonitrile (2×40 mL). The wet cake was partially dried under high vacuum and then transferred to a 75° C. vacuum oven and dried to constant weight to afford 2-(3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(cyclopropylsulfonyl)azetidin-3-yl)acetonitrile phosphate (12.0 g, 14.2 g theoretical, 84.5% yield) as white solids. For Phosphate: 1H NMR (DMSO-d6, 500 MHz) δ 12.13 (br. s, 1H), 9.20 (br. s, 3H), 8.94 (s, 1H), 8.70 (s, 1H), 8.47 (s, 1H), 7.61 (dd, 1H, J=3.4, 2.3 Hz), 7.07 (dd, 1H, J=3.6, 1.6 Hz), 4.65 (d, 2H, J=9.1 Hz), 4.28 (d, 2H, J=9.7 Hz), 3.68 (s, 2H), 2.82 (m, 1H), 1.01 (m, 4H) ppm; 13C NMR (DMSO-d6, 125 MHz) δ 152.9, 151.6, 150.0, 140.6, 130.3, 127.7, 122.9, 117.3, 113.8, 100.7, 59.7, 57.1, 27.6, 25.4, 4.9 ppm; C17H20N7O6PS (MW, 481.42; C17H17N7O2S for free base, MW, 383.43), LCMS (EI) m/e 384 (M++H). | 84.5% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Source: European Chemicals Agency (ECHA)
License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.
License URL: https://echa.europa.eu/web/guest/legal-notice
Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446
Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
| Transportation | Store at 2~8°C, away from light. |
| HS Code | |
| Storage | Store at 2~8°C, away from light. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 383.434 |
| logP | 0.366 |
| HBA | 9 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 128.94 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Ilunocitinib is a safe, effective, and convenient Janus kinase (JAK) inhibitor specifically designed to manage pruritus (itchiness) associated with allergic dermatitis, as well as to control atopic dermatitis (AD) in dogs aged 12 months and older. As a JAK inhibitor, Ilunocitinib works by interfering with the JAK-STAT signaling pathway, which is critical in mediating inflammation and immune responses in conditions like allergic and atopic dermatitis. By inhibiting this pathway, Ilunocitinib helps to reduce inflammation and alleviate the intense itching that often accompanies these conditions. Atopic dermatitis in dogs is a chronic skin disease that can cause severe discomfort due to persistent itching and inflammation, leading to skin lesions, infections, and a decreased quality of life. Traditional treatments, such as corticosteroids, often come with long-term side effects, making the development of targeted therapies like Ilunocitinib a significant advancement in veterinary medicine. Ilunocitinib offers a more targeted approach to managing the disease by directly inhibiting the pathways involved in the inflammatory response without broadly suppressing the immune system. Its safety profile and ease of administration make it a practical option for long-term management, improving the well-being of affected dogs and offering relief from the burdens of chronic dermatitis. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |