Indinavir CAS#: 150378-17-9; ChemWhat Code: 122584
Identification
| Product Name | Indinavir |
| IUPAC Name | (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N–tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide |
| Molecular Structure | 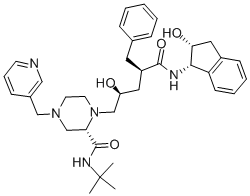 |
| CAS Registry Number | 150378-17-9 |
| NACRES | NA.77 |
| Synonyms | Crixivan hydrate, L-735,524 hydrate, MK-639 hydrate, indinavir, Indinavir, (1(1S,2R),5(S))-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-(2-(((1,1-dimethylethyl)amino)carbonyl)-4-(3-pyridinylmethyl)-1-piperazinyl)-2-(phenylmethyl)-D-erythro-pentonamide, (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-(2-methyl-2-propanyl)-4-(3-pyridinylmethyl)-2-piperazinecarboxamide, (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide, (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]carbamoyl}butyl]-N-tert-butyl-4-(pyridin-3 ylrnethyl)piperazine-2-carboxarnide, (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-l-yl]carbamoyl}butyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide |
| Molecular Formula | C36H47N5O4 |
| Molecular Weight | 613.8 |
| InChI | InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 |
| InChI Key | CBVCZFGXHXORBI-PXQQMZJSSA-N |
| Canonical SMILES | CC(C)(C)NC(=O)C1CN(CCN1CC(CC(CC2=CC=CC=C2)C(=O)NC3C(CC4=CC=CC=C34)O)O)CC5=CN=CC=C5 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2011/245283 | Methods for predicting the response to statins | 2011 |
| US2004/116692 | Reductive alkylation of saturated cyclic amines | 2004 |
Physical Data
| Appearance | White to off-white powder |
| Solubility | H2O: ≥15 mg/mL |
| Melting Point, °C | Solvent (Melting Point) | Comment (Melting Point) |
| 167.5 – 168 | ethyl acetate | Crystallization with 0.65 Mol(s) H2O |
| Type (Optical Rotatory Power) | Concentration (Optical Rotatory Power) | Solvent (Optical Rotatory Power) | Optical Rotatory Power, deg | Wavelength (Optical Rotatory Power), nm | Temperature (Optical Rotatory Power), °C |
| [alpha] | 0.0133 g/100ml | CHCl3 | 24.1 | 589 | 22 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | aq. phosphate buffer, dimethylsulfoxide | 37 | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-Dioleoylphosphatidylserine, dioleoylphosphatidylcholine |
| Further physical properties of the complex | aq. phosphate buffer, dimethylsulfoxide | 37 | 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine |
| IR spectrum of the complex | CHCl3 | acrylic acid |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | CDCl3 | |
| 1H, 13C | chloroform-d1 | 1H NMR (400 MHz, CDCl3) δ 8.53 (dd J=1.6), 8.52 (d, J=2.0), 7.68 (bs), 7.60 (d, J=7.9, 2.0), 7.29 (m), 7.23 (m), 1.18-7.09 (m), 6.19 (d, J=8.5), 5.27 (dd, J=8.5, 4.8), 4.27 (m), 3.92 (br s), 3.81 (m), 3.49 (s), 3.12 (t, J=3.6), 3.02 (dd, J=16.7, 5.2), 2.98-2.47 (m), 2.34 (br s), 1.97, 1.56 (m), 1.50 (br s), 1.34 (s). 13C NMR (100 MHz, CDCl3) δ 175.0, 169.4, 151, 149, 140.4, 140.3, 139.9, 137, 133, 129.1, 128.5, 127.9 126.7, 123.9, 126.5, 125.1 123.4, 73.0, 65.8, 64.1, 61.4, 60, 57.4, 54.6, 52.7, 51.1, 47.9, 46.5, 39.6, 39.1, 38.1, 29.0. | |
| Chemical shifts | 13C | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | CHCl3 | 3019 – 1366 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | methanol | Remark: first derivative spectrum, concentration dependence | ||
| methanol | Remark: first derivative spectrum | 273 | 9.284 |
Route of Synthesis (ROS)
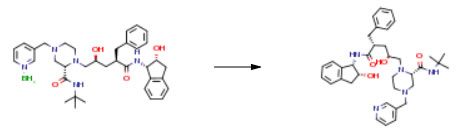
| Conditions | Yield |
| With methanol; hydrogen; platinum(IV) oxide In tetrahydrofuran for 3h; Experimental Procedure PtO2 (87 mg, 0.38 mmol) was added to the organic solution containing indinavir and its borane complex, whereupon the red-brown oxide immediately converted to very fine platinum black. Methanol (10 mL) was added and a mild evolution of hydrogen occurred over a period of about one hour. The batch was aged an additional two hours, whereupon HPLC analysis showed cleavage of the borane complex to be complete. The batch was then concentrated to remove THF, methanol and trimethoxyborate, and the concentrated batch was then diluted with isopropyl acetate. The batch was heated to 70° C., saturated with water and allowed to slowly cool to 0-5° C. to promote crystallization. The product was removed by filtration and washed with isopropyl acetate. Yield of 1=2.16 g, 92percent at a purity of 100percent by HPLC. 1H NMR (400 MHz, CDCl3) δ 8.53 (dd J=1.6), 8.52 (d, J=2.0), 7.68 (bs), 7.60 (d, J=7.9, 2.0), 7.29 (m), 7.23 (m), 1.18-7.09 (m), 6.19 (d, J=8.5), 5.27 (dd, J=8.5, 4.8), 4.27 (m), 3.92 (br s), 3.81 (m), 3.49 (s), 3.12 (t, J=3.6), 3.02 (dd, J=16.7, 5.2), 2.98-2.47 (m), 2.34 (br s), 1.97, 1.56 (m), 1.50 (br s), 1.34 (s). 13C NMR (100 MHz, CDCl3) δ 175.0, 169.4, 151, 149, 140.4, 140.3, 139.9, 137, 133, 129.1, 128.5, 127.9 126.7, 123.9, 126.5, 125.1 123.4, 73.0, 65.8, 64.1, 61.4, 60, 57.4, 54.6, 52.7, 51.1, 47.9, 46.5, 39.6, 39.1, 38.1, 29.0. | 92% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
| Acute Toxicity | LD50 Oral – Rat – > 5,000 mg/kg LD50 Oral – Mouse – > 5,000 mg/kg LD50 Oral – Dog – > 640 mg/kg Remarks: Gastrointestinal disturbance Inhalation: No data available Dermal: No data available LD50 Intraperitoneal – Rat – > 5,000 mg/kg LD50 Intraperitoneal – Mouse – > 5,000 mg/kg LD50 Intraperitoneal – Dog – > 640 mg/kg Remarks: Gastrointestinal disturbance |
Other Data
| Transportation | Not dangerous goods |
| Under room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under 2-8°C away from light |
| Shelf Life | 1 year |
| Market Price |
| Use Pattern |
| Indinavir CAS#: 150378-17-9 may be used in HIV-related cell signaling studies. |
| Indinavir CAS#: 150378-17-9 is an antiviral HIV protease inhibitor. Indinavir suppresses the replication of HIV and is an important component of antiretroviral therapy for initial treatment of HIV infection. It is known to cause renal and urologic toxicity. |
| reducing HIV- 1 reservoir in combination with antibody or a fragment that specifically binds to interferon-alpha/beta receptor to inhibit type I interferon signaling |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
