Lauryl Methacrylate (LMA) CAS 142-90-5; ChemWhat Code: 1411493
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114656358 | Method for preparing olefin-containing ester compound under catalysis of deep eutectic solvent | 2022 |
| CN114524789 | Method for synthesizing 3, 3-disubstituted isobenzofuran-1 (3H)-ketone with enantioselectivity | 2022 |
| CN114702517 | Application of chitosan Schiff base loaded bivalent copper material in preparation of beta-boryl ester | 2022 |
| CN106831665 | Method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone | 2017 |
| JP2016/6032 | COSMETICS HAVING COPOLYMER | 2016 |
Physical Data
| Appearance | Colorless to yellow liquid |
| Melting Point, °C |
| -7 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 130 – 140 | 10 |
| 167 | 10 |
| 142 | 4 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.87 | 4 | 20 |
| 0.8755 | 20 | |
| 0.8717 | 4 | 25 |
| 0.8735 | 4 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 101 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Spectrum | 1H | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | neat (no solvent) | |
| Bands, Spectrum | ||
| ATR (attenuated total reflectance), Bands, Spectrum | 25 |
Route of Synthesis (ROS)
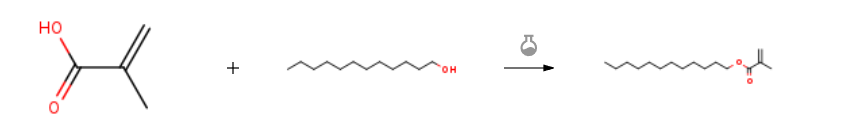
Route of Synthesis (ROS) of Lauryl Methacrylate (LMA) CAS142-90-5
| Conditions | Yield |
| With choline chloride; toluene-4-sulfonic acid; hydroquinone at 100℃; for 6h; | 94% |
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 4h; | 86% |
| With phosphoric acid; (p-tolueneslfonic acid, sulfosalicylic acid, resin KU-2×8); hydroquinone In toluene | |
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 4.16667h; Cooling with ice; Experimental Procedure According to the previous reported work, the reduction of lauricacid ester by lithium aluminum hydride (LAH) was done to producethe target lauryl alcohol [45]. In an ice bath system, dropwiseof a solution of lauryl alcohol (10 mmol) dissolved in dry DCM wasadded to a solution of DCC (11 mmol) and methacrylic acid(10 mmol) in DCM (20 mL) with continuous stirring for 10 mins.The reaction was stirred for 4 hrs at room temperature. After this time, the reaction was filtrated to remove dicyclohexyl urea, thefiltrate was concentrated under vacuum, and the LMA productwas purified using silica gel as an adsorbent (eluent: DCM) in columnchromatography to yield lauryl methacrylate [46]. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P271, P304+P340, P319, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 254.413 |
| logP | 6.616 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 13 |
| Matching Veber Rules | 1 |
| Quantitative Results | ||
| 1 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Emulsion and composition comprising a fluorohydrocarbon compound and a method for preparing such an emulsion and composition | |
| 2 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | SURFACE-ACTIVE n- ALKYL SULFO(METHYL)PROPIONATES | |
| 3 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 4 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| 5 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Stability of monomer emulsion droplets and implications for polymerizations therein | |
| 6 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Macromolecular surfactants for miniemulsion polymerization | |
| 7 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Preparation and nonlinear optical response of novel palladium-containing micellar nanohybrids |
| Use Pattern |
| Lauryl Methacrylate (LMA) CAS 142-90-5 is a commonly used surfactant and emulsifier that has found wide application in various fields. Firstly, Lauryl Methacrylate (LMA) CAS 142-90-5 in the personal care and cleaning agents industries, methyl laurate methacrylate is used as an excellent detergent and foaming agent, providing good cleaning performance and foamability. Additionally, it is often used in the production of personal care products such as shampoo, conditioner, body wash, soap. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
