m-Phenylenediamine CAS#: 108-45-2; ChemWhat Code: 23876
Identification
| Product Name | m-Phenylenediamine |
| IUPAC Name | benzene-1,3-diamine |
| Molecular Structure | 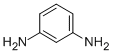 |
| CAS Registry Number | 108-45-2 |
| EINECS Number | 203-584-7 |
| MDL Number | MFCD00007799 |
| Beilstein Registry Number | 471357 |
| Synonyms | m-phenylenediamine, 1,3-phenylenediamine |
| Molecular Formula | C6H8N2 |
| Molecular Weight | 108.143 |
| InChI | InChI=1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2 |
| InChI Key | WZCQRUWWHSTZEM-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=CC(=C1)N)N |
Physical Data
| Appearance | Colorless needle crystal |
| Vapor Density | 3.7 (vs air) |
| Vapor Pressure | 0.62 mm Hg ( 100 °C) |
| Flash Point | >230 °F |
| Water Solubility | 350 g/L (25 ºC) |
| Pka | 5.11, 2.50(at 20℃) |
| Melting Point, °C | Solvent (Melting Point) |
| 65 | toluene, cyclohexane |
| 64 – 67 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 140 | 10 |
| 146 | 22 |
| 282 – 284 | 760 |
| Sublimation, °C | Pressure (Sublimation), Torr |
| 100 – 110 | 4 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.055 | 4 | 125 |
| 1.077 | 4 | 100 |
| 1.084 | 4 | 90 |
| 1.1389 | 15 | 15 |
| 1.1337 | 25 | 25 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.62558 | 656.3 | 57.7 |
| 1.6339 | 589 | 57.7 |
| 1.67617 | 434 | 57.7 |
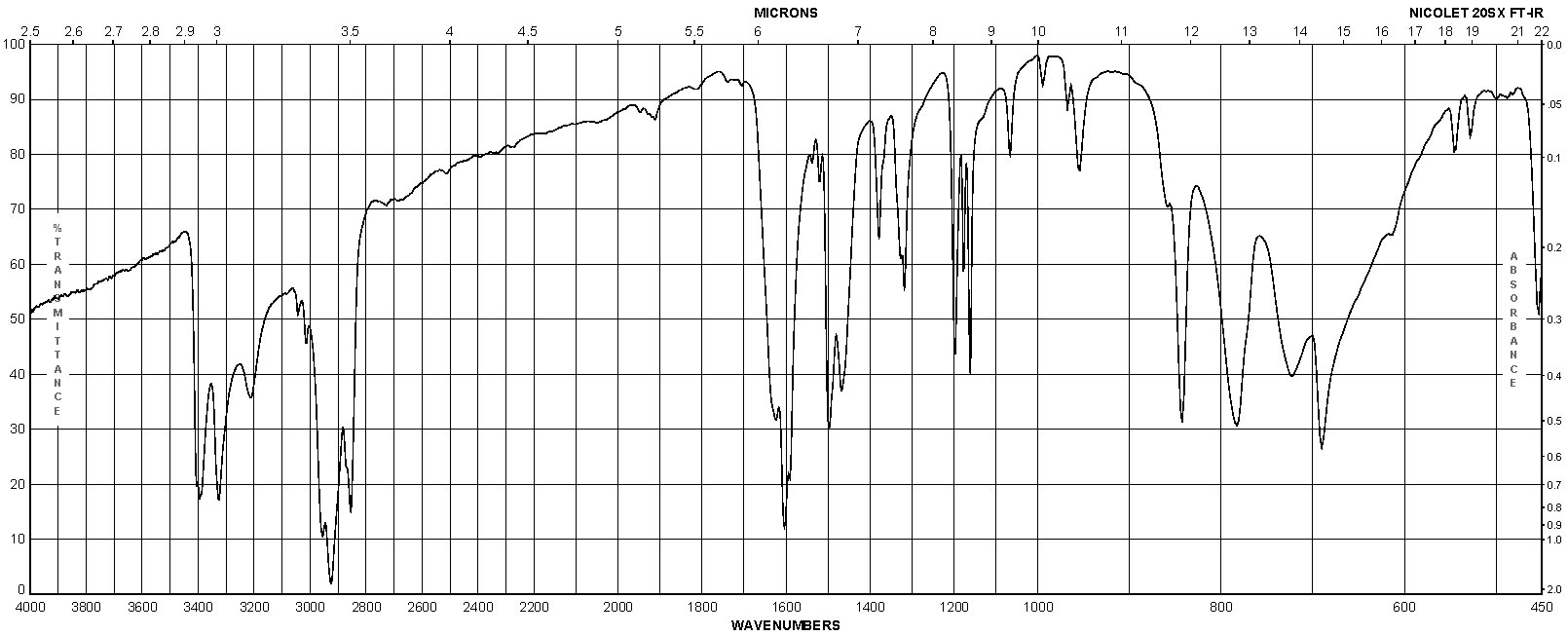
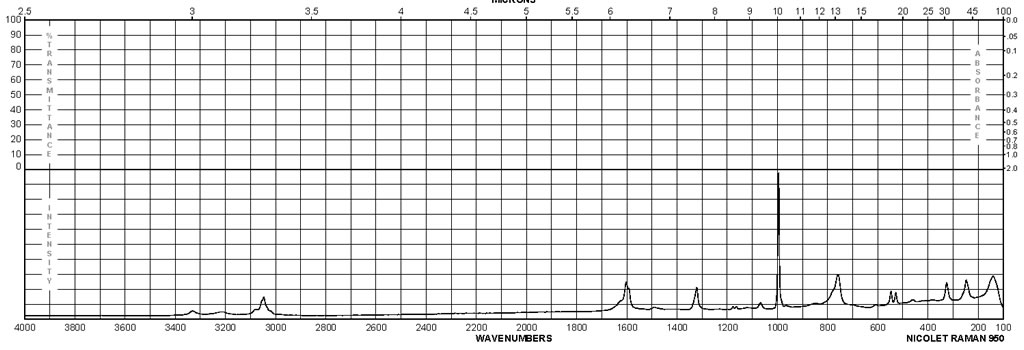
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
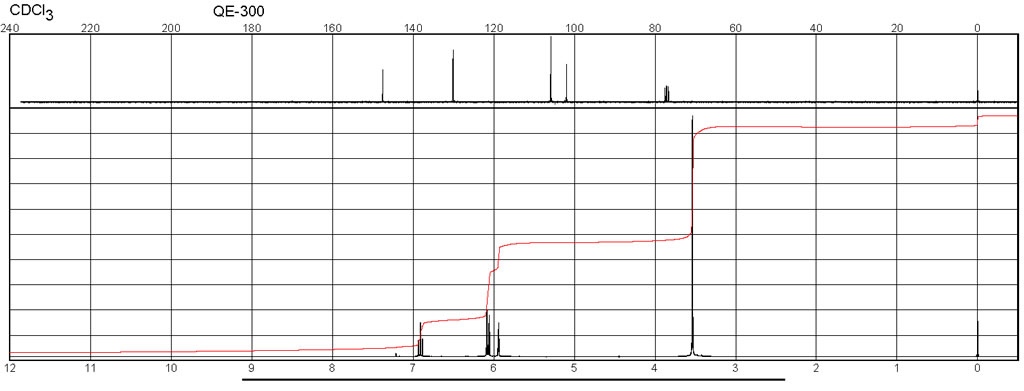
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 150 |
| Chemical shifts | 1H | chloroform-d1 | 24.84 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.84 | 75 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| acetonitrile | 215, 295 | 58100, 4900 | ||
| Spectrum | ethanol | 200 – 250 nm | ||
| Absorption maxima | ethanol | 292 | 2300 | |
| Spectrum | ethanol | 250 – 450 nm | ||
| Absorption maxima | H2O | 289, 238, 210 | 1995, 7244, 32359 | |
| Absorption maxima | methanol | 292.5, 242.5, 213.5 | 2291, 7079, 30199 |
Route of Synthesis (ROS)

| Conditions | Yield |
| With sodium tetrahydroborate In water at 20℃ for 1h | 100% |
| With hydrogen; sodium fluoride In methanol at 39.84℃ for 2.17 | 100% |
| With hydrogen In ethanol at 20℃ under 760.051 Torr for 3h chemoselective reaction Experimental Procedure General procedure: In this work, the following of nitroaromatic compoundswere used:The liquid-phase hydrogenation reaction of nitroaromaticcompounds can be presented as:It was carried out at room temperature under an atmosphericpressure of H2. A sample of the catalyst(50 mg) was placed in a round-bottomed threeneckedflask and the system was purged with hydrogenfor 30 min. A solution of the substrate in ethanol 0.2 M(or in tetrahydrofuran in the case of using o- andp-dinitrobenzene as a substrate) was then poured intothe reactor by means of a feed cock of the reactionmixture. The concentration of platinum in the reactionmixture was 0.85 mol %. The reaction was carriedout with vigorous stirring on a magnetic stirrer at a rateof 900 rpm in the monitoring mode by means of gasliquidchromatography until the peak of the startingcompound disappeared on the chromatogram. At theend of the experiment, the stirring was stopped; thecatalyst was separated from the reaction mixture bycentrifugation.Analysis of the reaction products was performedusing GC with a Chromatek Crystal 5000.2 chromatographwith a flame ionization detector (FID) and acapillary column CR-5 (2 mm × 25 m) at a temperatureof 190°C. The peaks were identified on the basis of the experimentally obtained retention times of individualcompounds (nitrobenzene, o-dinitrobenzene,p-dinitrobenzene, p-hydroxynitrobenzene, p-nitroacetophenone,nitrocyclohexane, aniline, o-phenylenediamine,p-phenylenediamine, p-hydroxyaniline,p-nitrophenylethanol, aminocyclohexane). Thestructure and purity of the products obtained wereconfirmed by NMR spectroscopy. The 1H and 13CNMR spectra were recorded on a Bruker Avance 300spectrometer in solvents CDCl3 and DMSO-d6. | 100% |
| With hydrazine hydrate In ethanol at 85℃ for 0.416667h | 99% |
| With borane-ammonia complex In methanol; water at 20℃ for 0.0833333h | 99% |
| With ammonia borane; Pd/MIL-101 In methanol; water at 20℃ for 0.05h | 99% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H331: Toxic if inhaled [Danger Acute toxicity, inhalation] H341: Suspected of causing genetic defects [Warning Germ cell mutagenicity] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P321, P322, P330, P333+P313, P337+P313, P361, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: III; UN Number: 1673 |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 7.5/kg |
| Use Pattern |
| m-Phenylenediamine CAS 108-45-2 may be used in the synthesis of the following: • intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures • extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls • series of terpolymers, via chemical oxidative polymerization • thin film composite (TFC) membranes based polyamide • TFC reverse osmosis (RO) membranes |
| m-Phenylenediamine (MPD) is an aromatic diamine. It copolymerizes with iso- or terephthaloyl chloride and 3,5-diaminobenzoic acid or 2,4-diaminobenzene sulfonic acid (or sulfonate) to afford copolyamides by low temperature solution polymerization. MPD has been reported to be formed selectively during the hydrogenation of m-dinitrobenzene in the presence of bimetallic Ni-Pt catalysts supported on carbon. Cyclic voltammetric method has been proposed to study the electrochemical copolymerization of aniline withMPD in sulfuric acid solution. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| DuPont | http://www.dupont.com/ |
| Zhejiang Longsheng Group Co.,Ltd.(浙江龙盛集团股份有限公司)(China) | http://www.longsheng.com |
| 江苏天嘉宜化工有限公司(China) | http://www.tianjiayichem.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |