N-(2-Bromoethyl)phthalimide CAS#:574-98-1; ChemWhat Code: 1411447
Identification
| Product Name | N-(2-Bromoethyl)phthalimide |
| IUPAC Name | 2-(2-bromoethyl)isoindole-1,3-dione |
| Molecular Structure | 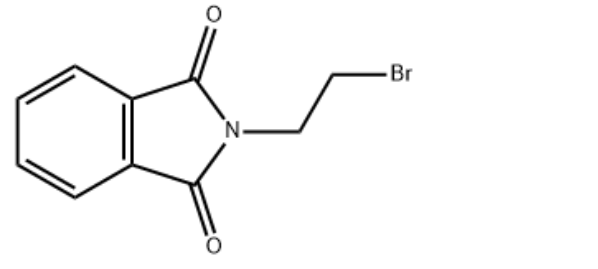 |
| CAS Registry Number | 574-98-1 |
| EINECS Number | 209-379-9 |
| MDL Number | MFCD00005902 |
| Beilstein Registry Number | 148736 |
| Synonyms | N-(2-BROMOETHYL)PHTHALIMIDE 574-98-1 2-(2-bromoethyl)isoindoline-1,3-dione 1H-Isoindole-1,3(2H)-dione, 2-(2-bromoethyl)- 2-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione 2-(2-bromoethyl)isoindole-1,3-dione 2-(Bromoethyl)phthalimide 1-Bromo-2-phthalimidoethane beta-Phthalimidoethyl bromide MFCD00005902 Phthalimide, N-(2-bromoethyl)- NSC 2688 2-Phthalimidoethyl bromide n(2-bromoethyl)phthalimide .beta.-Bromoethylphthalimide 2-(2-Bromo-ethyl)-isoindole-1,3-dione N-(2-bromoethyl) phthalimide .beta.-Phthalimidoethyl bromide N-(2-Bromoethyl-d4)phthalimide 2-(2-bromoethyl)-2,3-dihydro-1H-isoindole-1,3-dione 2-(2-bromoethyl)-isoindole-1,3-dione beta-Bromoethylphthalimide 2-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione; 1-Bromo-2-phthalimidoethane; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-ethyl bromide; 2-(2-Bromoethyl)-2,3-dihydro-1H-isoindole-1,3-dione N-2-Bromoethylphthalimide N-(2-Bromoethyl)-phthalimide EINECS 209-379-9 N-Bromoethylphthalimide phthalimidoethyl bromide 2-(2-bromoethyl)-1H-isoindole-1,3-dione N-(bromoethyl)phthalimide 2-(2-bromoethyl)benzo[c]azoline-1,3-dione N-(bromoethyl)-phthalimide n(2-bromoethyl) phthalimide N-(2-Bromoethyl)phthalimid SCHEMBL53287 2-(2-Bromoethyl)phthalimide N-beta-Bromoethyl-phthalimide N-(2-Bromoethyl)-phthalimid N-(2- bromoethyl)phthalimide N-(2-bromo-ethyl)phthalimide N-(Beta-bromoethyl)phthalimide CHEMBL595355 N-(2-bromo ethyl)-phthalimide N-(2-bromo-ethyl)-phthalimide N-[2-(bromo)ethyl]phthalimide DTXSID0060357 NSC2688 N-(.beta.-Bromoethyl)phthalimide ALBB-017759 NSC-2688 N-(2-Bromoethyl)phthalimide, 95% AC-165 STK291118 AKOS000119699 1H-Isoindole-1, 2-(2-bromoethyl)- CS-W008910 2-(2-Bromo-ethyl)isoindole-1,3-dione 2-(2-bromoethyl) isoindoline-1,3-dione AM808252 AS-11033 BP-13404 SY002569 DB-002815 B0597 FT-0629110 EN300-17894 1H-Isoindole-1,3(2H)-dione,2-(2-bromoethyl)- A831470 AE-641/00791032 2-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione # Q-201420 Z57069240 F3380-0002 |
| Molecular Formula | C10H8BrNO2 |
| Molecular Weight | 254.08 |
| InChI | InChI=1S/C10H8BrNO2/c11-5-6-12-9(13)7-3-1-2-4-8(7)10(12)14/h1-4H,5-6H2 |
| InChI Key | CHZXTOCAICMPQR-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=CN=C1)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN104447498 | A phthalimide derivative and its preparation method and application | 2017 |
| CN103373951 | Lapatinib process for the preparation of intermediates | 2016 |
| US2013/195879 | OXADIAZOLE INHIBITORS OF LEUKOTRIENE PRODUCTION FOR COMBINATION THERAPY | 2013 |
| EP1741709 | Heteroaryl-substituted amides comprising a saturated linker group, and their use as pharmaceuticals | 2007 |
Physical Data
| Appearance | Off white crystalline powder |
| Melting Point, °C | Solvent (Melting Point) |
| 83 | ethanol |
| 80 – 83 | |
| 81 – 84 | |
| 85 | |
| 151.3 – 152.5 | |
| 80 – 83 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 167 | 6 |
| 178 | 8 |
| 186 | 10 |
| 208 | 20 |
| 318 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 |
| Chemical shifts | 13C | chloroform-d1 | 126 |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 300 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 75 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | |
| Reflection spectrum, Bands | ||
| Intensity of IR bands, Bands, Spectrum | potassium bromide | |
| in KBr | ||
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | aq. buffer | |
| Spectrum | CHCl3 | 245 – 340 nm |
Route of Synthesis (ROS)
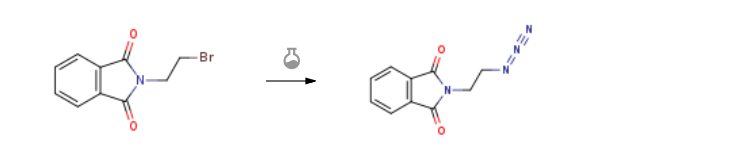
Route of Synthesis (ROS) of N-(2-Bromoethyl)phthalimide CAS 574-98-1
| Conditions | Yield |
| With sodium azide; potassium iodide In water; acetone at 20℃; for 60h; Inert atmosphere; | 92% |
| With sodium azide In N,N-dimethyl-formamide at 20℃; for 12h; | 91% |
| With sodium azide In N,N-dimethyl-formamide for 18h; Inert atmosphere; Reflux; | 90% |
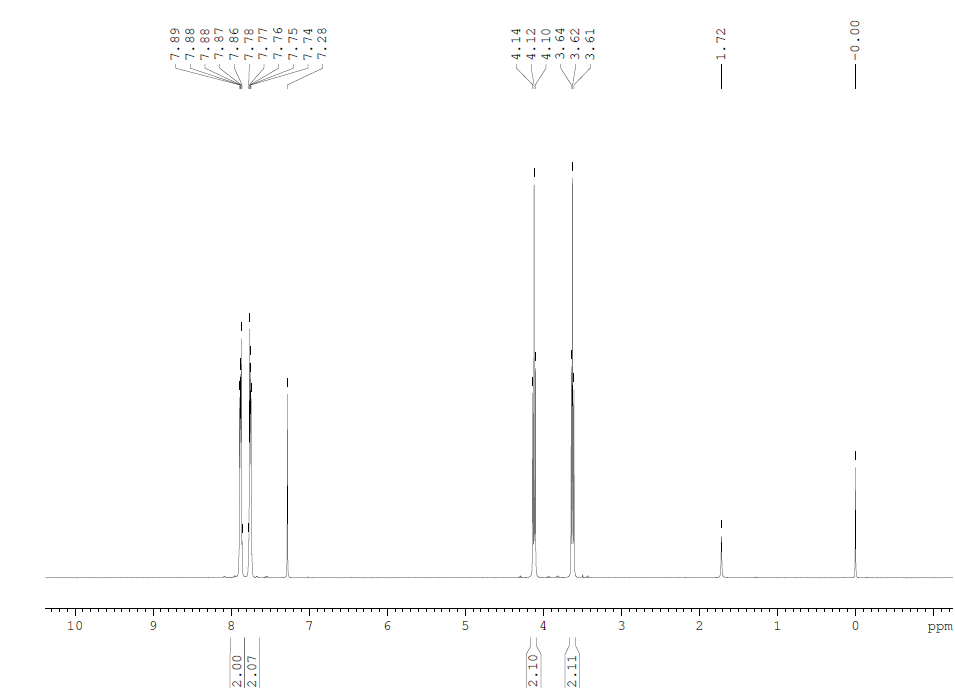
| Experimental Procedure A mixture of 2.6 g of N-(2-bromoethyl)phthalimide (3,10.2 mmol), 0.87 g of NaN3 (13.4 mmol), in 30 mL of dry DMF was refluxed for 18 h under argon. The mixture was evaporated to dryness; the residue was dissolved in 50 mL of dichloromethane and washed subsequently with 2×50 mL of water, 50 mL of brine, dried over MgSO4, filtered, concentrated and dried under vacuum to give pure compound 5 (2.12 g, 90%) as pearly white solid.1H NMR (CDCl3, 300 MHz, 25 °C) δ=7.85 (m, 2H, Ar-H), 7.70 (m, 2H, Ar-H), 3.88 (t, J=6 Hz, 2H, CH2), 3.56 (t, J=6 Hz, 2H, CH2).13C NMR (CDCl3, 75 MHz, 25 °C) δ=168.4 (ArC), 134.6 (ArCH), 132.2 (ArC), 123.8 (ArCH), 49.3 (CH2), 37.2 (CH2). |
Safety and Hazards
No data available
Other Data
| Transportation | Store in room temperature for long time; Protect from light. |
| Store in room temperature for long time; Protect from light. | |
| HS Code | |
| Storage | Store in room temperature for long time; Protect from light. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 254.083 |
| logP | 2.237 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.38 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Quantitative Results | ||
| 1 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | NOVEL ISOQUINOLINE DERIVATIVES OR SALTS THEREOF | |
| 2 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | FUROISOQUINOLINE DERIVATIVES, PROCESS FOR PRODUCING THE SAME AND USE THEREOF | |
| 3 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | DELTA-AMINO-GAMMA-HYDROXY-OMEGA-ARYL-ALKANOIC ACID AMIDES | |
| 4 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Novel thiourea derivatives and the pharmaceutical compositions containing the same | |
| 5 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | INSULIN DERIVATIVES CONJUGATED WITH STRUCTURALLY WELL DEFINED BRANCHED POLYMERS | |
| 6 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 7 of 79 | Biological material | Bioactivities present |
| Reference | ||
| 8 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 9 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 10 of 79 | Comment (Pharmacological Data) | Bioactivities present |
| Use Pattern |
| N-(2-Bromoethyl)phthalimide CAS#:574-98-1 is used in Pharmaceutical Intermediates,N-Substituted Maleimides, Succinimides |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |