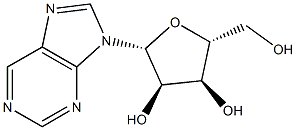
NUCLEOSIDE PHOSPHORYLASE CAS#: 9030-21-1; ChemWhat Code: 1293590
Identification
Physical Data
| Appearance | White amorphous powder |
Spectra
No data available
Route of Synthesis (ROS)
No data available
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Store at -20°C for long time, sealed and away from light. |
| Shelf Life | 2 years |
| Market Price |
| Use Pattern |
| NUCLEOSIDE PHOSPHORYLASE CAS#: 9030-21-1 a type of enzyme, is primarily involved in the metabolism of purine nucleotides. Its main function is to catalyze the transfer of a phosphate group between two adenylic acid (adenylate) molecules, forming an adenylic acid and an adenosine diphosphate (ADP) molecule. And Purine-Nucleoside Phosphorylase (PNP) functions in various cellular processes, including energy transfer, purine metabolism, and signal transduction. These functions are essential for maintaining normal cellular physiology and adapting to changes in the cellular environment. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |