O-Phosphorylethanolamine CAS#: 1071-23-4; ChemWhat Code: 33032
Identification
| Product Name | O-Phosphorylethanolamine |
| IUPAC Name | 2-aminoethyl dihydrogen phosphate |
| Molecular Structure | 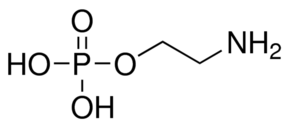 |
| CAS Registry Number | 1071-23-4 |
| EINECS Number | 213-988-5 |
| MDL Number | MFCD00008178 |
| Beilstein Registry Number | 1758916 |
| Synonyms | 2-aminoethyl dihydrogen phosphate;CAS Number: 1071-23-4 |
| Molecular Formula | C2H8NO4P |
| Molecular Weight | 141.063 |
| InChI | InChI=1S/C2H8NO4P/c3-1-2-7-8(4,5)6/h1-3H2,(H2,4,5,6) |
| InChI Key | SUHOOTKUPISOBE-UHFFFAOYSA-N |
| Canonical SMILES | C(COP(=O)(O)O)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2020/117755 | COMPOSITIONS AND METHODS FOR TREATING BIOFILMS | 2020 |
| EP1477493 | Isotope coded affinity tags | 2004 |
| EP1090630 | SKIN NORMALIZING AGENTS | 2001 |
| EP1210933 | SKIN NORMALIZING AGENTS | 2002 |
| US6610835 | SPHINGOLIPID DERIVATIVES AND THEIR METHODS OF USE | 2003 |
Physical Data
| Appearance | White to off-white crystalline powder |
| Boiling Point | 335.8±44.0 °C(Predicted) |
| Melting Point, °C | Solvent (Melting Point) |
| 232 – 233 | |
| 235 – 236 | |
| 236 – 238 | |
| 238.5 – 240 | aq. ethanol |
| 235 | H2O, methanol, diethyl ether |
| 240 | H2O, ethanol |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.497 | 589 | 22 |
| Density, g·cm-3 | Type (Density) |
| 1.562 | crystallographic |
| crystallographic |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Comment (Association (MCS)) | Partner (Association (MCS)) |
| Stability constant of the complex with … | Ca | ||
| Stability constant of the complex with … | Mg | ||
| Stability constant of the complex with … | Mn | ||
| Association with compound | H2O | silver (1+) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
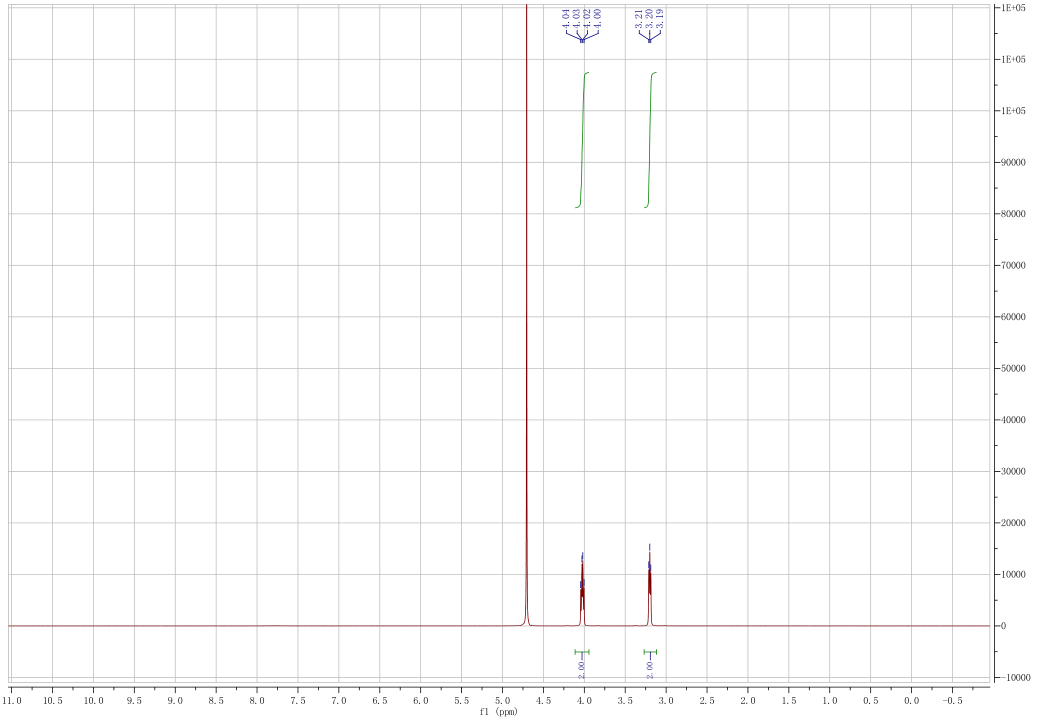
| Chemical shifts, Spectrum | 31P | 26.84 | 162 | |
| Chemical shifts | 1H | water-d2 | 24.84 | 400.1 |
| Chemical shifts | 31P | water-d2 | 161.9 | |
| Chemical shifts, Spectrum | 1H | water-d2 | 19.84 | 300 |
| COSY (Correlation Spectroscopy), Spectrum | 1H,1H | water-d2 | 19.84 | 300 |
| HSQC (Heteronuclear Single Quantum Coherence), Spectrum | 1H,13C | water-d2 | 19.84 | |
| Chemical shifts, Spectrum | 13C | water-d2 | 19.84 | 70 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | nujol | |
| Bands | nujol | 3300 – 1150 cm**(-1) |
| Bands | KBr | 2920 – 768 cm**(-1) |
| Bands | ||
| IR | ||
| Spectrum | KBr | 5000 – 667 cm**(-1) |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), time-of-flight mass spectra (TOFMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), high resolution mass spectrometry (HRMS), gas chromatography mass spectrometry (GCMS), spectrum |
| spectrum |
| liquid chromatography mass spectrometry (LCMS), tandem mass spectrometry, time-of-flight mass spectra (TOFMS), high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| Tandem mass spectrometry, ESI (Electrospray ionisation), Negative ion spectroscopy, Spectrum |
| ESI (Electrospray ionisation), Spectrum |
| Description (ESR Spectroscopy) | Temperature (ESR Spectroscopy), °C |
| Spectrum | -133.16 |
| ESR |
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: 2-aminoethyl dihydrogen phosphate With potassium tert-butylate In methanol at 20℃; for 0.5h; Cooling with ice; Inert atmosphere; Stage #2: pyridoxal 5′-phosphate With potassium tert-butylate In methanol at 0 – 20℃; for 0.5h; Inert atmosphere; Stage #3: With sodium tetrahydroborate In methanol at 0℃; for 4h; Reflux; Darkness; Inert atmosphere; Experimental Procedure 1 4.2.1. General procedure 1: reductive amination between PLP and selected amines General procedure: To an ice cooled solution of the selected amine (0.1 mmol) in dry CH3OH (3 mL) was added KOtBu (2 equiv) and the mixture was stirred at rt for 30 min (Solution A). Simultaneously, KOt-Bu (2 equiv/mol of PLP) was added to a solution of PLP (1.3 eq.) in CH3OH (3 mL) at 0°C and the mixture was stirred at rt for 30 min(Solution B). Solution A was then added dropwise to solution B at 0°C and the mixturewas refluxed in the dark for 3 h, cooled to 0°C and treated with NaBH4 (1.3 equiv). After stirring at rt for 1 h, the reaction mixture was acidified by dropwise addition of 6 M aq. HCl and the solvent was evaporated to dryness to give a residue, which was purified as indicated below. | 79% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314 (93.62%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 8; Packaging Group: II; UN Number: 3261 |
| Under the room temperature and away from light | |
| HS Code | 292219 |
| Storage | Under the room temperature and away from light |
| Market Price | USD 1200/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 141.064 |
| logP | -2.504 |
| HBA | 5 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 102.59 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 9.3 | amount | 70 | pg/ml | ||
| 6.6 | percentage increase | Active | Proliferative | ||
| 5.52 | Km (Michaelis constant) | = | 3 | µM | |
| 5 | Km (Michaelis constant)(Michaelis constant) | = | 9.9 | µM | |
| 4.62 | Kd (dissociation constant)(Equilibrium dissociation constant) | = | 24.1 | µM | |
| 4.27 | Km (Michaelis constant) | = | 54.2 | µM | |
| 4 | percentage decrease | Active |
| Quantitative Results | ||
| 1 of 4 | Target | Phosphoethanolamine N-methyltransferase [Caenorhabditis elegans]:Wild |
| Biological material | Caenorhabditis elegans | |
| Assay Description | Specificity constant of the compound towards Caenorhabditis elegans phosphoethanolamine N-methyltransferase expressed in Escherichia coli Rosetta II (DE3) pLysS cells upon incubation in 0.1 M Hepes, pH 8 for 7 min at 30 degree C measured as kcat/km | |
| Results | kcat/Km not calculated | |
| Measurement | kcat/Km | |
| 2 of 4 | Target | Phosphatase, Orphan 1 [human]:Wild |
| Substance action on target | Radioligand (/ligand) | |
| Biological material | human | |
| Assay Description | Maximum velocity of the compound against recombinant human PHOSPHO1 upon incubation in presence of 2 mM Mg2+ at 37 degree C using continuous coupled assay | |
| Results | Vmax not calculated | |
| Measurement | Vmax | |
| 3 of 4 | Target | Phosphatase, Orphan 1 [human]:Wild |
| Biological material | human | |
| Assay Description | Specific phosphatase activity of the compound against recombinant human PHOSPHO1 (3 ug/ml) in presence of 20 mM TBS, pH 7.2 upon incubation at 37 degree C | |
| Results | Activity not calculated | |
| 4 of 4 | Results | inhibitory activity on femoral bone marrow cells of ddY male mice |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 3.9 | inhibition rate | Active | antineoplastic agent | ||
| 3.89 | EC50 | 127.7 | μM | Oxidative Stress | |
| 3.75 | EC50 | 177.3 | μM | Oxidative Stress | |
| 3.72 | EC50 | 190.4 | μM | Oxidative Stress | |
| 3.29 | inhibition rate(inhibition of induced toxicity) | 33 | % | Oxidative Stress | |
| 3 | inhibition rate | Active | antineoplastic agent | ||
| 3 | inhibition rate | Active | antineoplastic agent |
| Use Pattern |
| O-Phosphorylethanolamine CAS#: 1071-23-4 is also used as the Dietary supplement. |
| O-Phosphorylethanolamine CAS#: 1071-23-4 is also used as food additives. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | http://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |