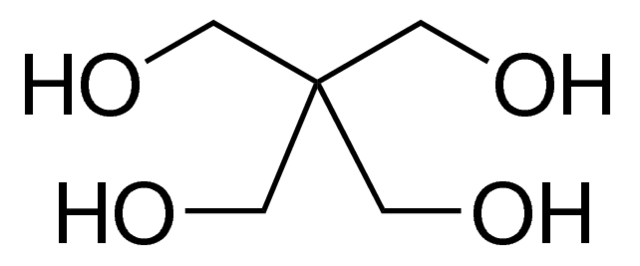
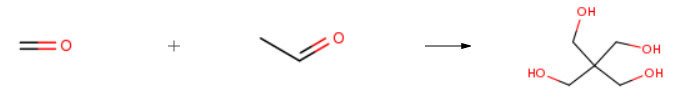
Stage #1: formaldehyd; acetaldehyde With sodium hydroxide In water
Stage #2: With formic acid In water pH=5.7; Product distribution / selectivity;
Experimental Procedure
Pentaerythritol was produced by a reaction between formaldehyde and acetaldehyde at a molar ratio of 5.0:1.0. Then, 3.08 parts by weight of acetaldehyde were added to an aqueous solution containing 10.51 parts by weight of formaldehyde and 7.47 parts by weight of an aqueous solution containing 45 percent sodium hydroxide. The molar ratio of wateπacetaldehyde was 66:1. The reaction was exothermic and the temperature was permitted to rise to 5O0C. The reaction mixture was neutralized with formic acid to a pH of 5.7. The pentaerythritol yield was about 90 percent calculated on acetaldehyde, which corresponds to 8.58 parts by weight of pentaerythritol.The reaction mixture was evaporated to a dryness of 62 percent by weight and thereafter cooled to 350C. Crystals of pentaerythritol thereby formed were separated off. The remaining process solution was treated separately to extract additional pentaerythritol and sodium formate.The pentaerythritol crystals were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 45 percent by weight. The solution was purified through active coal treatment and a subsequent ion exchanger. Thereafter the solution was cooled to 84°C and crystals of monopentaerythritol were precipitated. The crystals were separated off and 2.15 parts by weight of monopentaerythritol with a purity of 99.5 percent were obtained after drying. This corresponds to 25 percent of the total amount of pentaerythritol obtained. The purity was measured by gas chromatography.The remaining amount of pentaerythritol, constituting 6.43 parts by weight, was processed into technical pentaerythritol.Example 2The process according to example 1 was repeated with the difference that the crystals of pentaerythritol were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 55 percent by weight, and that the solution after the purification step was cooled to 9O0C. Thereby pure monopentaerythritol was obtained in an amount corresponding to 40 percent of the total amount of pentaerythritol obtained.Example 3Pentaerythritol was produced by a reaction between formaldehyde and acetaldehyde at a molar ratio of 6.0:1.0. Then, 3.08 parts by weight of acetaldehyde were added to an aqueous solution containing 12.61 parts by weight of formaldehyde and 7.47 parts by weight of an aqueous solution containing 45 percent sodium hydroxide. The molar ratio of water:acetaldehyde was 64:1. The reaction was exothermic and the temperature was permitted to rise to 5O0C. The reaction mixture was neutralized with formic acid to a pH of 5.7. The pentaerythritol yield was about 92 percent calculated on acetaldehyde, which corresponds to 8.76 parts by weight of pentaerythritol.The reaction mixture was evaporated to a dryness of 62 percent by weight and thereafter cooled to 350C. Crystals of pentaerythritol thereby formed were separated off. The remaining process solution was treated separately to extract additional pentaerythritol and sodium formate. The pentaerythritol crystals were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 45 percent by weight. The solution was purified through active coal treatment and a subsequent ion exchanger. Thereafter the solution was cooled to 7O0C and crystals of monopentaerythritol were precipitated and 5.43 parts by weight of monopentaerythritol with a purity of 99.6 percent were obtained after drying. This corresponds to 62 percent of the total amount of pentaerythritol obtained.The remaining amount of pentaerythritol, constituting 3.43 parts by weight, was processed into technical pentaerythritol.Example 4The process according to example 3 was repeated with the difference that the crystals of pentaerythritol were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 55 percent by weight, and that the solution after the purification step was cooled to 780C. Thereby pure monopentaerythritol was obtained in an amount corresponding to 66 percent of the total amount of pentaerythritol obtained.Example 5The process according to example 3 was repeated with the difference that the crystals of pentaerythritol were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 35 percent by weight, and that the solution after the purification step was cooled to 620C. Thereby pure monopentaerythritol was obtained in an amount corresponding to 55 percent of the total amount of pentaerythritol obtained.Example 6Pentaerythritol was produced by a reaction between formaldehyde and acetaldehyde at a molar ratio of 9.0:1.0. Then, 3.08 parts by weight of acetaldehyde were added to an aqueous solution containing 18.92 parts by weight of formaldehyde and 7.47 parts by weight of an aqueous solution containing 45 percent sodium hydroxide. The molar ratio of wateπacetaldehyde was 59:1. The reaction was exothermic and the temperature was permitted to rise to 500C. The reaction mixture was neutralized with formic acid to a pH of 5.7. The pentaerythritol yield was about 93 percent calculated on acetaldehyde, which corresponds to 8.86 parts by weight of pentaerythritol.The reaction mixture was evaporated to a dryness of 62 percent by weight and thereafter cooled to 350C. Crystals of pentaerythritol thereby formed were separated off. The remaining process solution was treated separately to extract additional pentaerythritol and sodium formate.The pentaerythritol crystals were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 45 percent by weight. The solution was purified through active coal treatment and a subsequent ion exchanger. Thereafter the solution was cooled to 54°C and crystals of monopentaerythritol were precipitated and 7.44 parts by weight of monopentaerythritol with a purity of 99.7 percent were obtained after drying. This corresponds to 84 percent of the total amount of pentaerythritol obtained.The remaining amount of pentaerythritol, constituting 1.42 parts by weight, was processed into technical pentaerythritol.Example 7The process according to example 6 was repeated with the difference that the crystals of pentaerythritol were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 35 percent by weight, and that the solution after the purification step was cooled to 46°C. Thereby pure monopentaerythritol was obtained in an amount corresponding to 80 percent of the total amount of pentaerythritol obtained.Example 8The process according to example 6 was repeated with the difference that the crystals of pentaerythritol were dissolved into a clear and warm solution in a water-based mother liquor containing pentaerythritol, to a dryness of 55 percent by weight, and that the solution after the purification step was cooled to 600C. Thereby pure monopentaerythritol was obtained in an amount corresponding to 86 percent of the total amount of pentaerythritol obtained.The crystallization temperature depends on the dryness at the dissolving but also on the molar ratio between formaldehyde and acetaldehyde since the molar ratio determines the composition of the pentaerythritol that is dissolved. Generally the crystallization temperature is highest at the lowest molar ratio and for each molar ratio the crystallization temperature is highest at the highest dryness. At each molar ratio the crystallization temperature and the dryness can be combined to obtain monopentaerythritol of high purity. | 90% |