Perinaphthenone CAS#: 548-39-0; ChemWhat Code: 1411552
Identification
Physical Data
| Appearance | Yellow solid |
| Melting Point, °C | Solvent (Melting Point) |
| 144 – 145 | |
| 155 – 157 | |
| 148 – 149 | |
| 149 – 151 | |
| 155 – 156 | benzene |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the adsorbed molecule | quartz | ||
| UV/VIS spectrum of the complex | CDCl3 | nile blue A perchlorate, O2 | |
| Stability constant of the complex with … | H2O | β-cyclodextrin | |
| Stability constant of the complex with … | H2O | β-hydroxypropyl-cyclodextrin, molar substitution 0.85 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 100 | |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | CD3CN |
| Bands | CHCl3 |
| Bands | KBr |
| Spectrum | gas |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | ||
| Spectrum | acetonitrile | |
| Spectrum | aq. phosphate buffer | |
| Spectrum | methanol | |
| Spectrum | methanol | 204, 248, 361 |
Route of Synthesis (ROS)
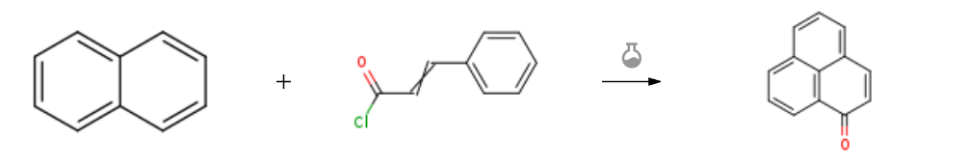
Route of Synthesis (ROS) of Perinaphthenone CAS 548-39-0
| Conditions | Yield |
| Stage #1: naphthalene With aluminum (III) chloride In dichloromethane for 0.0833333h; Cooling with ice; Stage #2: cinnamoyl chloride In dichloromethane at 20℃; for 0.833333h; | 76% |
| With carbon disulfide; aluminium trichloride | |
| Experimental Procedure Naphthalene (0.64 g, 5 mmol) and anhydrous aluminum chloride (1.4 g, 5.5 mmol) were dissolved in dichloromethane (10 mL) and stirred in an ice bath for 5 min, then solution of cinnamoyl chloride (0.83 g, 5 mmol) in 2.5 ml dichloromethane was added dropwise to the flask during 30 min and reacted at room temperature for 20 min. After that, the reaction solution was rotary evaporated under vacuum condition and purified with silica gel chromatography using petroleum ether/ethyl acetate (10: 1) as eluting solvent to afford phenalenone as a yellow solid with a yield of 76% (0.40 g). m.p. 144-145 oC. 1H NMR (400 MHz, Chloroform-d) δ 8.59 (d, J = 7.6 Hz, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.77 – 7.67 (m, 3H), 7.55 (t, J = 7.6 Hz, 1H), 6.71 (d, J = 9.8 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 185.14, 137.17, 132.90, 132.63, 131.84, 131.61, 131.51, 130.24, 129.30, 129.25, 127.89, 127.15, 126.61. HRMS (ESI): m/z calcd. for C13H9O+ [M + H] +: 181.0653; found: 181.0654 |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (50%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (50%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (50%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (50%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Room Temperature and Long-term storage 2-8°C |
| HS Code | |
| Storage | Room Temperature and Long-term storage 2-8°C |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 180.206 |
| logP | 3.092 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 17.07 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 44 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Fluorescent haloalkyl derivatives of reporter molecules well retained in cells | |
| 2 of 44 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Hydrolyzable fluorescent substrates and analytical determinations using same | |
| 3 of 44 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 4 of 44 | Comment (Pharmacological Data) | Bioactivities present |
| Reference |
| Use Pattern |
| Perinaphthenone CAS#: 548-39-0 as an important intermediate in the synthesis of various pharmaceuticals, including anticancer drugs, antibiotics, and antimalarial drugs. And it is extensively employed in photosensitive materials due to its ability to absorb ultraviolet and visible light. Perinaphthenone is used in the production of organic semiconductor materials and is utilized as an antioxidant and preservative in cosmetics and personal care products. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |