Phenoxycycloposphazene CAS#: 1184-10-7; ChemWhat Code: 119635
Identification
| Product Name | Phenoxycycloposphazene |
| IUPAC Name | 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2λ5,4λ5,6λ5-triphosphacyclohexa-1,3,5-triene |
| Molecular Structure | 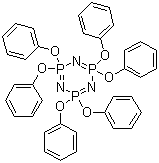 |
| CAS Registry Number | 1184-10-7 |
| EINECS Number | 208-127-2 |
| Synonyms | 2,2,4,4,6,6-hexaphenoxycyclotriphosphazene, 2,2,4,4,6,6-hexa-(phenoxo)cyclotri-λ5-phosphazatriene, 2,2,4,4,6,6-hexakisphenoxycyclotriphosphazene, hexaphenoxycyclotriphosphazatriene, hexa(phenoxy)cyclotriphosphazene, hexaphenoxycyclo(triphosphazene), hexaphenoxycyclotriphosphazene |
| Molecular Formula | C36H30N3O6P3 |
| Molecular Weight | 693.572 |
| InChI | InChI=1S/C36H30N3O6P3/c1-7-19-31(20-8-1)40-46(41-32-21-9-2-10-22-32)37-47(42-33-23-11-3-12-24-33,43-34-25-13-4-14-26-34)39-48(38-46,44-35-27-15-5-16-28-35)45-36-29-17-6-18-30-36/h1-30H |
| InChI Key | RNFJDJUURJAICM-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC=C(C=C1)OP2(=NP(=NP(=N2)(OC3=CC=CC=C3)OC4=CC=CC=C4)(OC5=CC=CC=C5)OC6=CC=CC=C6)OC7=CC=CC=C7 |
Physical Data
| Appearance | White to light yellow crystalline powder |
| Boiling Point | 280°C/0.1mmHg(lit.) |
| Melting Point, °C | Solvent (Melting Point) |
| 109 – 111 | ethanol |
| 113.4 | methanol |
| 110 – 111 | benzene, petroleum ether |
| 112 – 112.5 | heptane, benzene |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.2104 | 120 |
| 1.4076 – 1.4085 | 20 |
| 1.4083 | 20 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.558 | 589 | 25 |
| 1.564 | 589 | 120 |
| 1.5802 | 589 | 19 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d | 400 |
| Chemical shifts | 31P | chloroform-d1 | 145 |
| Chemical shifts | 13C | chloroform-d1 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | chloroform | 261 |
Route of Synthesis (ROS)
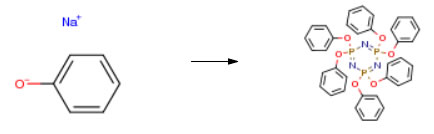
| Conditions | Yield |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In acetonitrile at 30 – 80℃ for 5h Experimental Procedure In the same reaction vessel, was added 1000g of acetonitrile, cooled to 30°C using a solid funnel was slowly added hexachlorocyclotriphosphazene 120g (0.345mol), 1 hour addition time, the final temperature of the feed 45°C, heated up to 80 deg.] C, began to reflux, 4 hours after the reaction by HPLC timing hexachloro cyclotriphosphazene residual content of 0.20percent, the reaction was complete. first atmospheric distillation of acetonitrile, to be distilled slowly, slowly open the vacuum distillation under reduced pressure the remaining acetonitrile recovered acetonitrile 980g.Acetonitrile evaporated after addition of 2percent sodium hydroxide solution and 600ml of toluene obtained in Step 1 was heated to 55°C, stirring for 1 hour, points to the water layer, and then separately with 600ml of 5percent sodium chloride solution and 600ml of pure water, and washed once by the same method of layering a final product to obtain a toluene solution. Toluene was distilled under reduced pressure to complete a total of recovered toluene 490g. After distillation of the residual material in toluene was added 300g of dry ethanol and heated to reflux for 1 hour, then cooled to 20 deg.] C and held for one hour, centrifuged, and dried 60 deg.] C for 5 hours to obtain a white hexaphenoxycyclotriphosphazene228g, HPLC content of 99.25percent is detected, a yield of 95.24percent, a chlorine ion content was 100ppm. | 95.24% |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine | |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In 1,4-dioxane; diethyl ether Heating | |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In tetrahydrofuran Inert atmosphere |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261-P305+P351+P338 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 8 dangerous goods |
| Phenoxycycloposphazene CAS#: 1184-10-7 should be kept under the room temperature and away from light | |
| HS Code | 292690 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 50/kg |
| Use Pattern |
| Phenoxycycloposphazene CAS#: 1184-10-7 can be used as an additive-tip fire retardant or halogen-free fire retardant in epoxy resin,copper clad plate ,LED lumlinous diode,powder paint,encapsulating material or polymer matenial. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
