Phosphonitrilic chloride trimer CAS#: 940-71-6; ChemWhat Code: 62299
Identification
| Product Name | Phosphonitrilic chloride trimer |
| IUPAC Name | 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2λ5,4λ5,6λ5-triphosphacyclohexa-1,3,5-triene |
| Molecular Structure | 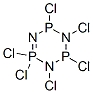 |
| CAS Registry Number | 940-71-6 |
| EINECS Number | 213-376-8 |
| MDL Number | MFCD00006474 |
| Synonyms | 940-71-6 CAS Number 940-71-6 CAS NO. 940-71-6 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine |
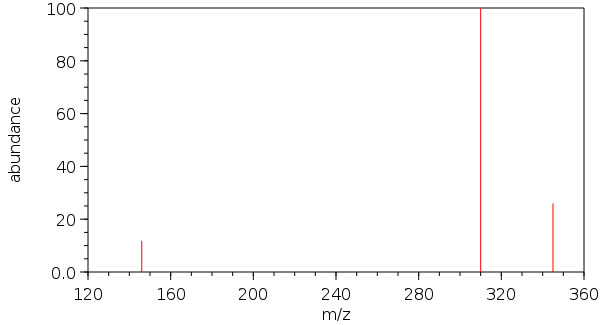
| Molecular Formula | Cl6N3P3 |
| Molecular Weight | 347.659 |
| InChI | InChI=1S/Cl6N3P3/c1-10(2)7-11(3,4)9-12(5,6)8-10 |
| InChI Key | UBIJTWDKTYCPMQ-UHFFFAOYSA-N |
| Canonical SMILES | N1=P(N=P(N=P1(Cl)Cl)(Cl)Cl)(Cl)Cl |
Physical Data
| Appearance | White crystalline powder |
| Melting Point, °C | Solvent (Melting Point) |
| 113.3 – 113.9 | Petroleum ether |
| 111 – 113 | n-heptane |
| 112 – 114 | light petroleum |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 127 | 13 |
| 116 – 117 | 11 |
| 256.6 | 760 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 2.131 | -173.16 |
| 1.98 | |
| 2.13 | 99.84 |
| Refractive Index | Wavelength (Refractive Index), nm | Comment (Refractive Index) |
| 1.619 | 589.3 | nα |
| 1.621 | 589.3 | nβ |
| 1.631 | 589.3 | nγ |
| Sublimation, °C | Pressure (Sublimation), Torr |
| 60 | 0.05 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
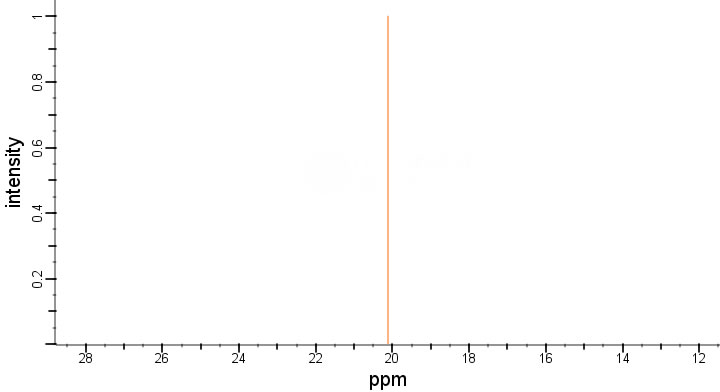
| Chemical shifts | 31P | chloroform-d1 | 400 | |
| Chemical shifts | 31P | chloroform-d1 | 24.84 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | potassium bromide | 25 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | ethanol, water | 200 |
| Spectrum | tetrahydrofuran | 175, 287 |
| Spectrum | hexane | 186 |
Route of Synthesis (ROS)
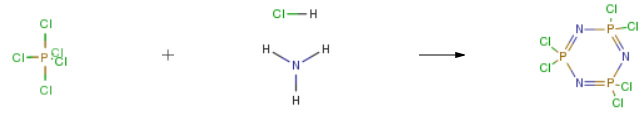
| Conditions | Yield |
| With pyridine; magnesium chloride In chlorobenzene at 80 – 132℃ Inert atmosphere Experimental Procedure 20.8 g (0.1 mol) of phosphorus pentachloride and 62.4 ml of anhydrous chlorobenzene were added to a 250 ml four-necked flask under nitrogen atmosphere, Slowly raised the temperature to 80 ° C, and stirred at 80 ° C for 1 to 2 hours until the phosphorus pentachloride was completely dissolved and stand-by. 5.88 g of ammonium chloride, 571 mg of magnesium chloride, 474 mg of pyridine and 62.4 ml of anhydrous chlorobenzene were placed in a 250 ml four-necked flask under nitrogen atmosphere, and slowly heated to a reflux state of 130 to 132 ° C under nitrogen atmosphere. The prepared phosphorus pentachloride chlorobenzene solution was slowly added dropwise to a 250 ml four-necked flask under reflux, and the dropping time was controlled to be not less than 4 hours. After the completion of the dropwise addition, stirring was continued under reflux for 1 to 2 hours; After cooling to room temperature, filtration, the mother liquid was concentrated to remove the solvent to obtain a crude compound hexachlorocyclotriphosphazene (referred to as “compound 2”).The crude compound 2 obtained above and 10.5 ml of petroleum ether (boiling range 60 to 90) were added to a 50 ml jacketed bottle, Slowly raised the temperature to 80 ° C, stirred at 80 ° C for 1 to 2 hours until the crude product 2 was completely dissolved, and let stand to separate and remove a small amount of oil. The petroleum ether solution was then extracted twice with 40 ml of 98percent concentrated sulfuric acid, and the concentrated sulfuric acid extract was diluted to 60percent concentrated sulfuric acid solution with 22.7 ml of deionized water to precipitate a crude product of gray hexachlorocyclotriphosphazene. Crude compound 2 and 10.5 ml of petroleum ether (boiling range 60-90) were added to a 50 ml jacketed flask, slowly warmed to 80 ° C, and stirred at 80 ° C for 1 to 2 hours until the product was completely dissolved. Then, the temperature was slowly lowered to 0 to 5 ° C for crystallization, and after filtration, 8.22 g of Compound 2 as white crystals was obtained, yield 71percent.The obtained compound was tested and found to be a cyclic hexachloro phosphazene compound having a specific crystal form. | 71% |
| Stage #1: ammonium chloride With pyridine; magnesium chloride In 1,1,2,2-tetrachloroethane at 145℃ for 12h Stage #2: phosphorus pentachloride In 1,1,2,2-tetrachloroethane at 145℃ Solvent Temperature Experimental Procedure Ammonia chloride, an inert solvent tetrachloroethane and a mixed catalyst of magnesium chloride and pyridine with a mass ratio of 1: 3 were added into the synthesis kettle, and the mixture was heated to 145 ° C. after being uniformly mixed,The use of variable frequency solid feeder within 12 hours of phosphorus pentachloride solid into the synthesis reactor, the amount of inert solvent tetrachloroethane phosphorus three times, phosphorus pentachloride, ammonium chloride,Mixed catalyst mass ratio of 1: 0.3: 0.08; maintaining the temperature of 145 ° C to the end of the reaction, phosphorus pentachloride complete reaction was completed, cooled to 40,Filtered out excess ammonium chloride and catalyst, the solvent was removed under reduced pressure solvent tetrachloroethane, cooled to 10 ° C and centrifuged to give hexachlorocyclotriphosphazene. | 61.78% |
| In chlorobenzene at 120 – 130℃ Inert atmosphere Reflux Experimental Procedure In 250mL three-necked flask with constant pressure dropping funnel and a magnetic stirring device, followed by 16.0g of fine grinding120mL of ammonium chloride and chlorobenzene; Weigh 34.0g of phosphorus pentachloride in 100mL beaker, add 80mL of chlorobenzene,Heating Stir until completely dissolved phosphorus pentachloride chlorobenzene, quickly transferred to a constant pressure funnel, and make insulation measuresShi prevent precipitation of phosphorus pentachloride from chlorobenzene solution. Turn the heating apparatus under a nitrogen atmosphere until the solution temperature reached three-necked flask120-130 when to start uniform solution was added dropwise phosphorus pentachloride in chlorobenzene, was added dropwise in approximately 2.5h appropriate controls, dropWas completed, the temperature was raised to reflux, approximately of 2-3h reaction, heating was stopped, cooling, filtration, rotary evaporation, the solid recrystallized with petroleum etherJing, washed with ethanol, a white diamond crystal HCPP, a yield of 60.6percent. | 60.6% |
| zinc(II) oxide In 1,2-dichloro-benzene at 177℃ for 2h Product distribution / selectivity Experimental Procedure Example 22; First Step; A 100 ml four-neck flask equipped with a stirrer, a condenser, a dropping funnel and a thermometer was charged with 1.93 g (0.036 mol) of ammonium chloride having an average particle size of 2.1 μm, 0.041 g (0.5 mmol) of zinc oxide and 17 g of o-dichlorobenzene. Nitrogen flow was introduced into the flask. Part of the reaction solution was collected by a microsyringe and the moisture content was measured. As a result, the moisture content was 3.2.x.10-4 mole based on 1 mole of phosphorus pentachloride. Subsequently, while heating at an oil bath temperature of 177° C., a solution of 6.25 g (0.03 mol) of phosphorus pentachloride in 17 g of o-dichlorobenzene was added dropwise to the reaction system through the dropping funnel heated to 105° C. After completion of the addition, the reaction was performed for 2 hours. During the reaction, the moisture content in the reaction system was less than 3.2.x.10-4 mole based on 1 mole of phosphorus pentachloride. The reaction solution was used in the second step without filtration.; Example 23; First Step; A 100 ml four-neck flask equipped with a stirrer, a condenser, a dropping funnel and a thermometer was charged with 1.93 g (0.036 mol) of ammonium chloride having an average particle size of 2.1 μm, 0.041 g (0.5 mmol) of zinc oxide and 17 g of o-dichlorobenzene. Nitrogen flow was introduced into the flask. Part of the reaction solution was collected by a microsyringe and the moisture content was measured. As a result, the moisture content was 2.5.x.10-4 mole based on 1 mole of phosphorus pentachloride. Subsequently, while heating at an oil bath temperature of 177° C., a solution of 6.25 g (0.03 mol) of phosphorus pentachloride in 17 g of o-dichlorobenzene was added dropwise to the reaction system through the dropping funnel heated to 105° C. After completion of the addition, the reaction was performed for 2 hours. During the reaction, the moisture content in the reaction system was less than 2.5.x.10-4 mole based on 1 mole of phosphorus pentachloride. After completion of the reaction, the resultant was cooled to room temperature and unreacted ammonium chloride was removed by filtration under reduced pressure. The amount of zinc contained in the filtrate was 2.4.x.10-4 mole based on 1 mole of phosphonitrile dichloride.; Example 24; First Step; A 100 ml four-neck flask equipped with a stirrer, a condenser, a dropping funnel and a thermometer was charged with 1.93 g (0.036 mol) of ammonium chloride having an average particle size of 2.1 μm, 0.041 g (0.5 mmol) of zinc oxide and 17 g of o-dichlorobenzene. Nitrogen flow was introduced into the flask. Part of the reaction solution was collected by a microsyringe and the moisture content was measured. As a result, the moisture content was 1.9.x.10-4 mole based on 1 mole of phosphorus pentachloride. Subsequently, while heating at an oil bath temperature of 177° C., a solution of 6.25 g (0.03 mol) of phosphorus pentachloride in 17 g of o-dichlorobenzene was added dropwise to the reaction system through the dropping funnel heated to 105° C. After completion of the addition, the reaction was performed for 2 hours. During the reaction, the moisture content in the reaction system was less than 2.5.x.10-4 mole based on 1 mole of phosphorus pentachloride. After completion of the reaction, the resultant was cooled to room temperature and unreacted ammonium chloride was removed by filtration under reduced pressure.; Comparative Example 10; First Step; A 100 ml four-neck flask equipped with a stirrer, a condenser, a dropping funnel and a thermometer was charged with 1.93 g (0.036 mol) of ammonium chloride having an average particle size of 2.1 μm, 0.041 g (0.5 mmol) of zinc oxide and 17 g of o-dichlorobenzene. Nitrogen flow was introduced into the flask. Part of the reaction solution was collected by a microsyringe and the moisture content was measured. As a result, the moisture content was 1.9.x.10-4 mole based on 1 mole of phosphorus pentachloride. Subsequently, while heating at an oil bath temperature of 177° C., a solution of 6.25 g (0.03 mol) of phosphorus pentachloride in 17 g of o-dichlorobenzene was added dropwise to the reaction system through the dropping funnel heated to 105° C. After completion of the addition, the reaction was performed for 2 hours. During the reaction, the moisture content in the reaction system was less than 2.5.x.10-4 mole based on 1 mole of phosphorus pentachloride. After completion of the reaction, the resultant was cooled to room temperature and unreacted ammonium chloride was removed by filtration under reduced pressure and the resulting reaction solution was put in a 100 ml separatory funnel. 50 ml of distilled water was added thereto and the mixture in the separatory funnel was sufficiently shaken at room temperature and allowed to stand for a while to separate oil from water. After separating the dichlorobenzene phase, magnesium sulfate was added thereto and the mixture was stirred for 30 minutes. After removing magnesium sulfate by filtration, molecular sieve 4 A was added. The resultant was left overnight and then the molecular sieve 4 A was removed by filtration. The amount of zinc in the filtrate was 5.2.x.10-7 mole based on 1 mole of phosphonitrile dichloride. | |
| chlorobenzene | |
| In chlorobenzene at 140 – 160℃ | |
| With pyridine; zinc(II) chloride at 70 – 132℃ for 2h Temperature Sonication Large scale Experimental Procedure A preferable example is that the ammonium chloride is 2 kg, the phosphorus pentachloride is 6 kg, the zinc chloride is 25 g, the acid binding agent is 40 ml of pyridine, the reaction temperature is 70 ° C., The reaction time was 2 hours and the heating temperature was 132 ° C. Yet another preferred example is that the ammonium chloride is 2.2 kg, the phosphorus pentachloride is 6 kg, the zinc chloride is 28 g, the acid binding agent is 39.8 ml of pyridine, and the reaction temperature is 90 ° C., the reaction time was 1.9 hours, and the heating temperature was 130 ° C. As another example, the ammonium chloride is 5kg, the phosphorus pentachloride is 12kg, the zinc chloride is 59g, the acid binding agent is 81ml of pyridine, the reaction temperature is 120 , the reaction time Of 1.85 hours and the heating temperature was 133 ° C. For another example, the ammonium chloride is 3.1 kg, the phosphorus pentachloride is 9 kg, the zinc chloride is 38 g, the acid binding agent is 59 ml of pyridine and the reaction temperature is 148 ° C. The reaction The time was 1.8 hours and the heating temperature was 127 ° C. The above examples, tested, can reach more than 85percent or even 93percent to 96percent yield of the reaction product, namely hexachlorocyclotriphosphazene. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 8; Packaging Group: II; UN Number: 3260 |
| Under the room temperature and away from light | |
| HS Code | 292690 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 40/kg |
| Use Pattern |
| Phosphonitrilic chloride trimer CAS#: 940-71-6 can be used as a reagent for the synthesis of ″dandelion″ (spherical) dendrimers. Ring-opening polymerization, ligand and/or ligand precursor for transition metals, and the study of P-Cl bond substitution reactions are among the interesting uses for Phosphonitrilic chloride trimer CAS#: 940-71-6. |
| Phosphonitrilic chloride trimer CAS#: 940-71-6 can be used as an additive-tip fire retardant or halogen-free fire retardant in epoxy resin, copper clad plate, LED lumlinous diode, powder paint, encapsulating material or polymer matenial. Hexachloro cyclotriphosphazene high consumption of chlorine products, Phosphonitrilic chloride trimer CAS#: 940-71-6 can be used as pesticides, fertilizers, anticancer drugs, a phase transfer catalyst, a radical polymerization initiator, light stabilizers, antioxidants, flame retardants. Phosphonitrilic chloride trimer CAS#: 940-71-6 can also be synthesized by polymerization function broader organic – inorganic polymer material that can be used in catalytic materials, high temperature rubber, flame retardant materials, polymer electrolyte, the photoconductive polymer materials, nonlinear optical materials, bio-medical polymer materials , liquid crystal polymer, membrane, medical, military and other |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |