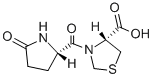
Pidotimod CAS#: 121808-62-6; ChemWhat Code: 87409
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP276752 | Derivative of thiazolidine-4-carboxylic acid, its preparation and pharmaceutical compositions containing it | 1988 |
| EP450352 | Liposome formulations of immunomodulating drugs for the topical and aerosol administrations | 1991 |
| EP572942 | Oral pharmaceutical compositions for specific colon delivery | 1993 |
| US5369131 | Oral, cutaneous and intravaginal pharmaceutical compositions in the form of foam | 1994 |
Physical Data
| Appearance | White crystalline powder,odorless |
| Melting Point, °C | Solvent (Melting Point) |
| 195 – 197 | |
| 193.99 | |
| 194 – 198 | |
| 195 – 198 | propan-2-ol, H2O |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.509 | 19.84 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | ||
| Spectrum | 1H | ||
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | |
| Spectrum | 1H | dimethylsulfoxide-d6 | 31.9 – 76.9 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands, Spectrum | |
| Spectrum | KBr |
| Bands | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
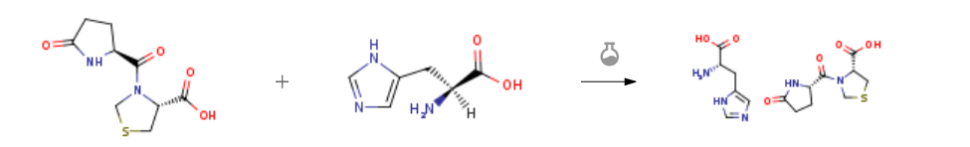
| In ethanol; water at 5℃; for 5h; Solvent; Temperature; Sonication; Experimental Procedure Take 0.50g of pidotimod and 40ml of absolute ethanol to form a suspension by sonication. After dissolving the other 0.31g of histidine and 4ml of purified water, slowly add the histidine solution to the aforementioned pidotimod mixture at room temperature. Suspended solution, shake well after adding, cool to 5, continue to stir and crystallize for 5h,Filter to obtain crystalline powder, rinse with absolute ethanol 2 to 3 times,5ml each time, dried under vacuum at 40°C for 10 hours to constant weight to obtain pidotimod histidine salt, crystalline powder, yield 95.2%, purity 99.97% (active ingredient pidotimod detection) | 95.2% |
Safety and Hazards
No data available
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 244.271 |
| logP | -1.007 |
| HBA | 6 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 112.01 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Derivative of thiazolidine-4-carboxylic acid, its preparation and pharmaceutical compositions containing it | |
| 2 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Toxicological evaluation of pidotimod | |
| 3 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | 3-L-(5-thioxo-L-prolyl)thiazolidine-4-carboxylic acid and derivatives therefrom, processes for the preparation thereof and pharmaceutical compositions containing them | |
| 4 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Pidotimod: The state of art | |
| 5 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Effects of pidotimod soluble powder and immune enhancement of Newcastle disease vaccine in chickens | |
| 6 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides | |
| 7 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Effects of Pidotimod on recurrent respiratory infections in children with down syndrome: A retrospective Italian study | |
| 8 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Laser therapy in cutaneous and genital warts: A review article | |
| 9 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Pidotimod injection with modified stability, and preparation method thereof | |
| 10 of 61 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Amantadine pidotimod carboxylate and preparation method and application thereof |
| Use Pattern |
| Pidotimod CAS#: 121808-62-6 is an immunomodulator suitable for patients with compromised immune function. It can be used for the prevention of acute infections, shorten the duration, and reduce the severity of the disease. It can be used as an adjunctive therapy during the acute infection period. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |