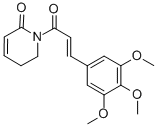
PIPERLONGUMINE CAS#: 20069-09-4; ChemWhat Code: 51049
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2022/153306 | ANTI-QUORUM SENSING, ANTI-BIOFILM, AND INFLAMMATION ATTENUATING COMPOUNDS, COMPOSITIONS, AND METHODS OF USING SAME | 2022 |
| WO2020/68425 | COMPOSITIONS AND METHODS FOR ENHANCING INSULIN SECRETION | 2020 |
| WO2019/54891 | TRPV2 ANTAGONISTS | 2019 |
| WO2019/103897 | DERIVATIVES OF PIPERLONGUMINE AND USES THEREOF | 2019 |
| WO2016/14625 | COMPOSITIONS AND METHODS FOR SELECTIVELY DEPLETING SENESCENT CELLS | 2016 |
Physical Data
| Appearance | White to off-white powder |
| Melting Point, °C |
| 120 – 121 |
| 122 – 123 |
| 156 – 158 |
| 125 – 127 |
| 124 |
| Density, g·cm-3 |
| 1.2 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | dimethyl sulfoxide | 24.84 | calf thymus DNA |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | 400 | |
| Chemical shifts, Spectrum | 13C | 100 | |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts | 13C | chloroform-d1 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | ||
| Bands, Spectrum | potassium bromide | |
| ATR (attenuated total reflectance), Bands |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | 230, 330 | ||
| Spectrum | aq. phosphate buffer | ||
| Spectrum | |||
| Spectrum | acetonitrile |
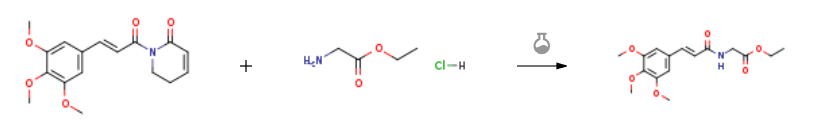
Route of Synthesis (ROS)
Route of Synthesis (ROS) of PIPERLONGUMINE CAS 20069-09-4
| Conditions | Yield |
| Stage #1: glycine ethyl ester hydrochloric acid With triethylamine In ethanol at 20℃; for 0.5h; Stage #2: (E)-1-(3-(3,4,5-trimethoxyphenyl)acryloyl)-5,6-dihydropyridin-2(1H)-one In ethanol at 20℃; for 1.5h; | 78% |
| Stage #1: glycine ethyl ester hydrochloric acid With triethylamine In ethanol at 20℃; for 0.5h; Stage #2: (E)-1-(3-(3,4,5-trimethoxyphenyl)acryloyl)-5,6-dihydropyridin-2(1H)-one In ethanol for 1.5h; Experimental Procedure To a stirred solution of glycine ethyl ester. HC1 (66.04 mg, 0.473 mmol) in ethanol (0.8 mL), triethylamine 48.2 mg, 0.473 mmol) was added and stirred for 30 min. To this SPL (50.0 mg, 0.157 mmol) was added and stirred at room temperature. The reaction was monitored by TLC (Hexane-ethyl acetate (1:1). Starting material consumed within 1.5 h. After completion, the reaction mixture was quenched with 5% aq. HC1 (10 mL) and extracted with ethyl acetate (2 x 20 mL), the combined organic layer was washed with water (1 x 20 mL), 5% NaHCOs solution (20 mL), water (2 x 20 mL), dried over anhyd. Na2SO4 and concentrated under reduced pressure. To the crude reaction mass, 2 mL of diethyl ether was added, and the reaction mass was filtered and dried to get pure product as white solid in 40 mg (yield 78%). | 78% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (50%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 317.342 |
| logP | 1.413 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 65.07 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | CYTOTOXIC PYRIDONE ALKALOIDS FROM PIPER ABORESCENS | |
| 2 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ALR2 INHIBITORS AND THEIR SYNTHESIS FROM A NATURAL SOURCE | |
| 3 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | A piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. | |
| 4 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Piperlongumine for Enhancing Oral Bioavailability and Cytotoxicity of Docetaxel in Triple-Negative Breast Cancer | |
| 5 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | METHOD OF TREATING MEDULLOBLASTOMA | |
| 6 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Piperlongumine induces G2/M phase arrest and apoptosis in cholangiocarcinoma cells through the ROS-JNK-ERK signaling pathway | |
| 7 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Fragmentation pattern of amides by EI and HRESI: Study of protonation sites using DFT-3LYP data | |
| 8 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Mild, Metal-Free and Protection-Free Transamidation of N-Acyl-2-piperidones to Amino Acids, Amino Alcohols and Aliphatic Amines and Esterification of N-Acyl-2-piperidones | |
| 9 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | An autophagy modifier screen identifies small molecules capable of reducing autophagosome accumulation in a model of CLN3-mediated neurodegeneration | |
| 10 of 264 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | COMPOSITIONS AND METHODS FOR TREATING RAS-MUTANT CANCERS |
| Use Pattern |
| piperlongumine-cas-20069-09-4 has been used in traditional medicine as an analgesic and anti-inflammatory agent, particularly in traditional Chinese medicine. It is believed to have potential benefits for certain conditions and dietary supplement. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |