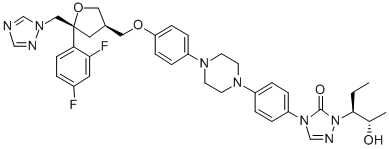
Posaconazole CAS#: 171228-49-2; ChemWhat Code: 41467
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/40363 | AZOLE ANALOGUES AND METHODS OF USE THEREOF | 2019 |
| US2019/388397 | COMPOSITIONS AND METHODS FOR THE TREATMENT AND PREVENTION OF NEUROLOGICAL DISORDERS | 2019 |
| US2014/303184 | PROCESS FOR THE PREPARATION OF A CHIRAL COMPOUND | 2014 |
| US2014/343285 | Process for the Preparation of Triazole Antifungal Drug, Its Intermediates and Polymorphs Thereof | 2014 |
Physical Data
| Appearance | white to off-white powder |
| Melting Point, °C | Solvent (Melting Point) |
| 168.2 | |
| 171.44 | |
| 168 – 171 | |
| 171.4 | acetone, water |
| 170.1 | |
| 171 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.321 | -173.16 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| MAS (Magic-Angle Spinning), Spectrum | 1H | |
| 2D-NMR, Spectrum | 1H, 13C | |
| MAS (Magic-Angle Spinning), Spectrum | 13C | |
| Chemical shifts, Spectrum | 1H | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | |
| Bands, Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| UV excited state absorption, Spectrum | methanol | 203 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogenchloride; water at 25 – 63℃; Temperature; | 95% |
| With hydrogenchloride; Pd/C; hydrogen In methanol; water at 50℃; under 750.075 Torr; for 2h; Temperature; Reagent/catalyst; Pressure; Sealed tube; Large scale; | 93.6% |
| With hydrogenchloride In water at 45 – 50℃; for 20h; Large scale; Experimental Procedure Add 95.5kg of concentrated hydrochloric acid to a 300L reactor, The compound 3 obtained in the previous step was added with stirring, and then the temperature was raised to 45-50°C to react for 20 hours. TLC (EA) detection showed that there was no spot on compound 3. The reaction is considered complete. After the reaction is complete, cool down to 20-30°C, Use 20%-30% sodium hydroxide solution 100.0-120.0kg to adjust PH to 9-10, temperature control≤30, crystallize at room temperature for 2h after completion, filter, The filter cake is rinsed with 10kg methanol, and the filter cake is air-dried at 40-45°C for 8 hours . Get 12.12kg. The yield was 84.43%. H-HNM see Figure 3. Refining of posaconazole 11.4kg of crude posaconazole was added to 90kg of methanol, heated to reflux for 30min, and 67.5kg of purified water was added dropwise while keeping warm. Cool down naturally to 20-25, keep incubating and crystallize for 2h, filter, rinse with 10kg of filter cake and methanol/water mixed solution, add ratio of filter cake to methanol/water mixed solution at 1:1, 40-45 blast drying The finished product was 10.48kg in 8h. The yield was 91.93%. | 91.93% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H319 (75%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H351 (20.83%): Suspected of causing cancer [Warning Carcinogenicity] H361 (97.92%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H372 (100%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H400 (95.83%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (95.83%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P260, P264, P264+P265, P270, P273, P280, P305+P351+P338, P318, P319, P337+P317, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 700.788 |
| logP | 5.721 |
| HBA | 11 |
| HBD | 1 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 111.79 |
| Rotatable Bond (RotB) | 12 |
| Matching Veber Rules | 1 |
| Quantitative Results | ||
| 1 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Crystalline antifungal polymorph | |
| 2 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Activity of SCH 56592 compared with those of fluconazole and itraconazole against Candida spp. | |
| 3 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes | |
| 4 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. | |
| 5 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans | |
| 6 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | CRYSTALLINE ANTIFUNGAL POLYMORPH | |
| 7 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | PROCESS FOR PREPARING POSACONAZOLE AND INTERMEDIATES THEREOF | |
| 8 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | New antifungal peptides. Synthesis, bioassays and initial structure prediction by CD spectroscopy | |
| 9 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Anti-leishmanial activity of betulin derivatives | |
| 10 of 2,228 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Steroid-transforming enzymes in fungi |
| Use Pattern |
| Posaconazole CAS#: 171228-49-2 is a second-generation triazole antifungal drug widely used for the prevention and treatment of invasive fungal infections. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |