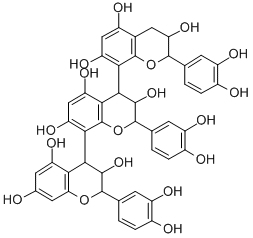
PROCYANIDIN C1 CAS#: 37064-30-5; ChemWhat Code: 98195
Identification
Physical Data
| Appearance | Brown powder |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | 23.84 | bovine milk β-lactoglobulin |
| Association with compound | 23.84 | bovine milk α-lactalbumin |
| Association with compound | 30.84 | bovine milk β-lactoglobulin |
| Association with compound | 30.84 | bovine milk α-lactalbumin |
| Association with compound | 37.84 | bovine milk β-lactoglobulin |
| Association with compound | 37.84 | bovine milk α-lactalbumin |
| Association with compound | bovine serum albumin |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | CD3OD | ||
| Chemical shifts | 13C | CD3OD | ||
| Chemical shifts | 1H | CD3OD | 400 | |
| Chemical shifts | 1H | CD3OD | -18.16 | 400 |
| Chemical shifts | 13C | CD3OD | -18.16 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Spectrum | potassium bromide |
| Description (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| methanol | 200, 280 |
| aq. phosphate buffer | 280 |
| water | 280 |
| methanol, water | 281 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogen; palladium(II) hydroxide/carbon In tetrahydrofuran; methanol; water under 750.075 Torr; for 1.33333h; Experimental Procedure 11.A Example 11; Preparation of Epicatechin (4β,8)-Oligomers from Benzyl-Protected Oligomers; A. Preparation of Epicatechin (4β,8)2-Trimer To a solution of 64.3 mg (33.0 μmol) of bis(5,7,3′,4′-tetra-O-benzyl)epicatechin (4β,8)-dimer in 5 mL of tetrahydrofuran were added 5 mL of methanol, 0.25 mL of water, and 57 mg of 20% Pearlman’s catalyst (Pd(OH)2/C). The mixture was stirred under 1 bar of hydrogen for 80 minutes and filtered over cotton. The filtration residue was washed two times with 10 mL of methanol. The combined filtrates were evaporated, and the residue was taken up in 10 mL of HPLC grade water. The solution was filtered and lyophilized to yield 32.4 mg (101%) of epicatechin (4β,8)2-trimer 6H2O as a fluffy, amorphous, off-white solid: [α]D+70.4°, [α]546+84.40 (MeOH, c 2.2 gL-1); (ref. 4c: [α]D+75.20, acetone, c 8.7 gL-1; ref. 4d: [α]578+90°, MeOH, c 2 gL-1; ref. 6: [α]D+76.40, acetone, c 8.6 gL-1; ref. 19b: []578+920, H2O, c 1.9 gL-1; ref. 19k: [α]D+800, MeOH, c 1.6 gL-1); 13C NMR (CD3OD, TMS; δ 60-85 region only) δ 79.73, 77.08, 73.47, 72.94, 66.84; MS (API/ES) m/z 865.4 (100%; calcd for [M-H]-: 865.2), 577.0 (6%), 288.9 (4%). Analysis: Calculated for C45H38O18.6H2O: C, 55.44; H, 5.17. Found: C, 55.71; H, 5.07. | 100% |
| With hydrogen; palladium dihydroxide In tetrahydrofuran; methanol | 95% |
| With hydrogen; palladium dihydroxide In tetrahydrofuran; methanol; water at 20℃; for 1h; | 95% |
| With 10 wt% Pd(OH)2 on carbon; hydrogen In tetrahydrofuran; methanol; water at 20℃; for 1h; | 92% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
| For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 866.786 |
| logP | 3.855 |
| HBA | 3 |
| HBD | 15 |
| Matching Lipinski Rules | 2 |
| Veber rules component | |
| Polar Surface Area (PSA) | 331.14 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 1 |
| Use Pattern |
| Procyanidin C1 CAS 37064-30-5, a complex polyphenolic compound, falls under the larger category of proanthocyanidins which are widely recognized for their potent antioxidant properties. These substances are found in a variety of plants, including fruits, vegetables, nuts, seeds, and bark, playing crucial roles in plant defense mechanisms against pathogens and environmental stressors. Among the diverse array of proanthocyanidins, Procyanidin C1 is particularly notable for its unique structure and significant health-promoting potentials. Chemically, Procyanidin C1 is a trimer, meaning it consists of three flavonoid units linked together. This structural complexity contributes to its strong antioxidant capacity, surpassing that of simpler flavonoids and even some vitamins known for their antioxidant properties, such as vitamin E and C. The antioxidant activity of Procyanidin C1 is essential in neutralizing free radicals, unstable molecules that can cause oxidative stress leading to cellular damage, aging, and various diseases. The health benefits associated with Procyanidin C1 are vast and have been the subject of numerous scientific studies. Research has shown that it can support cardiovascular health by improving blood circulation, reducing blood pressure, and preventing the oxidation of low-density lipoprotein (LDL) cholesterol, a key factor in the development of atherosclerosis. Moreover, its anti-inflammatory effects are crucial in mitigating chronic inflammation, a root cause of many chronic conditions, including cancer, diabetes, and neurodegenerative diseases. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |