Propylene glycol CAS#: 57-55-6; ChemWhat Code: 80788
Identification
| Product Name | Propylene glycol |
| IUPAC Name | propane-1,2-diol |
| Molecular Structure | 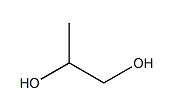 |
| CAS Registry Number | 57-55-6 |
| EINECS Number | 200-338-0 |
| MDL Number | MFCD00064272 |
| Beilstein Registry Number | 1340498 |
| Synonyms | propylene glycol, 1,2-Propanediol, Propyleneglycol;CAS Namber: 57-55-6;CAS No:.57-55-6;57-55-6;CAS#: 57-55-6; |
| Molecular Formula | C5H6N2 |
| Molecular Weight | 94.116 |
| InChI | InChI=1S/C3H8O2/c1-3(5)2-4/h3-5H,2H2,1H3 |
| InChI Key | DNIAPMSPPWPWGF-UHFFFAOYSA-N |
| Canonical SMILES | CC(CO)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2020/63220 | Microbicidal composition (by machine translation) | 2020 |
| CN111217856 | Pentacyclic phosphate compound as well as preparation method and application thereof (by machine translation) | 2020 |
| CN111320530 | Preparation method 1 – hydroxyl -2 -alkyl ketone (by machine translation) | 2020 |
| CN111333607 | Method for synthesizing apple ester compound (by machine translation) | 2020 |
Physical Data
| Appearance | Colorless, viscous and stable absorbent liquid |
| Solubility | miscible |
| Refractive index | n20/D 1.432(lit.) |
| Sensitivity | Hygroscopic |
| Melting Point, °C |
| 187 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 214 | |
| 188 | |
| 101 – 102 | 6.0006 |
| 187.6 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.43 | 589 | 24.99 |
| 1.4272 | 589 | 34.99 |
| 1.4312 | 589 | 24.99 |
| 1.42246 | 589 | 49.99 |
| 1.42591 | 589 | 39.99 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.0329 | 24.99 |
| 1.0356 | 24.99 |
| 1.0178 | 44.99 |
| 1.0256 | 34.99 |
| 1.0366 | 19.99 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption and desorption isotherms | acetonitrile | -196.16 | chromium based metal-organic frameworks |
| Further physical properties of the adsorbed molecule | -103.16 | Pd(111) | |
| Adsorption | water | 24.84 | ruthenium, hydrogen |
| Adsorption | water | 24.84 | ruthenium |
| Further physical properties of the adsorbed molecule | CDCl3 | 1 wt% Au/TiO2 | |
| Further physical properties of the adsorbed molecule | M41S mesoporous silica | ||
| Further physical properties of the adsorbed molecule | 0 – 40 | sol-gel porous glass |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |||
| Chemical shifts | 1H | 13C | water, water-d2 | ||
| Chemical shifts | 13C | water, water-d2 | |||
| Spectrum | 13C | dimethylsulfoxide-d6 | 100.6 | ||
| Spectrum | 1H | dimethylsulfoxide-d6 | |||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | |
| Intensity of IR bands, Bands, Spectrum | |
| Spectrum | |
| Bands, Spectrum | |
| ATR (attenuated total reflectance), Spectrum | |
| Bands | |
| ATR (attenuated total reflectance), Spectrum | neat liquid |
| Description (Mass Spectrometry) |
| time-of-flight mass spectra (TOFMS), gas chromatography mass spectrometry (GCMS), spectrum |
| gas chromatography mass spectrometry (GCMS), IT (ion trap), electron impact (EI), spectrum |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), spectrum |
Route of Synthesis (ROS)
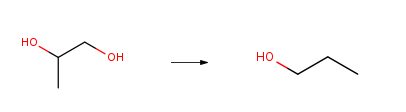
| Conditions | Yield |
| With [C6H3-2,6-(OP(tBu)2)2]IrH2; trifluorormethanesulfonic acid; water; hydrogen In 1,4-dioxane at 125℃; under 5171.62 Torr; Temperature; Pressure; Solvent; Autoclave; Experimental Procedure 11 Hydrogenation of 1,2 Propanediol. Hydrogenation of 1,2 Propanediol. Using (POCOP)IrH2 and trifluoromethanesulfonic acid, 1,2-propanediol (1,2 PD) was reduced to n-propanol in up to 95% yield in aqueous dioxane at 125° C. under 100 psi H2. The mild conditions of the reaction and the high selectivity observed are extraordinary. Even more remarkable is the stability of this catalyst to the aqueous environment. In fact, the presence of water is actually required to achieve high deoxygenation selectivity and hydrogenation efficiency, which also increased as the acid concentration in the reaction system was decreased. | 95% |
| With hydrogen In water at 210℃; under 30753.1 Torr; for 5h; Autoclave; Sealed tube; | |
| With hydrogen In water at 179.84℃; under 37503.8 Torr; for 12h; | 80 %Chromat. |
| at 315℃; Gas phase; |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 290532 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 76.0953 |
| logP | -0.748 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit |
| 2.55 | Km (Michaelis constant)(Michaelis-menten constant) | = | 2.8 | mM |
| 2.37 | EC50 | 325 – 3785 | mg/L | |
| 2.27 | LOAEC | 405 | mg/L | |
| 2.18 | EC50 | 500 | mg/L | |
| 2.04 | LC50 | 686 | mg/L | |
| 1.96 | Km (Michaelis constant)(Michaelis-menten constant) | = | 0.011 | M |
| Quantitative Results | ||
| 1 of 10 | Effect | antimicrobial agent |
| Assay Description | Bioassay : EXPERIMENTALS Experimental 1: Selection of Antimicrobial preservatives for Injectable Compound of Formula la. Study A (Large Antimicrobial preservative Screen) The efficacy of several different antimicrobial preservatives in combination with compound of Formula la and SBE-CD were investigated. Literature | |
| Results | title compound at 50% in combination with 5% sulfobutylether-p-cyclodextrin (SBE-CD) and formula 1a showed antimicrobial effect in USP test, European Pharmacopoeia, criteria A test and European Pharmacopoeia, criteria B test | |
| 2 of 10 | Effect | antibiotic agent |
| Biological material | Escherichia coli | |
| Assay Description | Effect : antibacterial Species : ATCC 29522 Example 1Synergistic Antimicrobial ActivityThis example provides the results for antimicrobial activity of components of exemplary invention’s compositions when the components were used alone (Tables 3A and 3B), or as a combination of two components (Tables 3A and 4), or of at least three components | |
| Results | log reduction (5.45 log inoculum) after treatment for 2/30/180 min: 1.83/2.24/2.55 (strawberry); 1.71/2.1/2.4 (spinach leaf) | |
| 3 of 10 | Effect | antibiotic agent |
| Biological material | Escherichia coli | |
| Assay Description | Effect : antibacterial Species : ATCC 43888 Example 1Synergistic Antimicrobial ActivityThis example provides the results for antimicrobial activity of components of exemplary invention’s compositions when the components were used alone (Tables 3A and 3B), or as a combination of two components (Tables 3A and 4), or of at least three components | |
| Results | log reduction (6.87 log inoculum) after treatment for 2/30/180 min: 2.23/2.69/4.47 (strawberry); 2.44/2.54/3.98 (spinach leaf) | |
| 4 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (25) with 5 menthol on permeation coefficient of propofol through rat skin; n = 3 | |
| Results | kp not calculated | |
| 5 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (29) with 1 lauric acid 1695 on permeation coefficient of propofol through rat skin; n = 3 | |
| Results | kp not calculated | |
| 6 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (25) with 1 oleic acid 1570 on permeation coefficient of propofol through rat skin; n = 3 | |
| Results | kp not calculated | |
| 7 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (29) with 1 lauric acid 1695 on flux of permeation of propofol through rat skin; n = 3 | |
| Results | Jss not calculated | |
| 8 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (25) with 1 lauric acid 1695 on permeation coefficient of propofol through rat skin; n = 3 | |
| Results | kp not calculated | |
| 9 of 10 | Biological material | rat |
| Assay Description | Permeation coefficient of the compound as enhancer through rat skin for 8 h at 20 concentration | |
| Results | kp not calculated | |
| 10 of 10 | Biological material | rat |
| Assay Description | Effect of combining the compound (25) with 5 oleic acid on permeation coefficient of propofol through rat skin; n = 3 | |
| Results | kp not calculated |
| Use Pattern |
| Propylene glycol CAS# 57-55-6 is a widely used basic material. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
