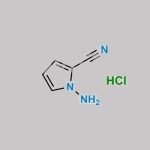
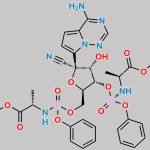
PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE CAS#: 159326-68-8; ChemWhat Code: 549058
Identification
| Product Name | PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE |
| IUPAC Name | pyrrolo[2,1-f][1,2,4]triazin-4-amine |
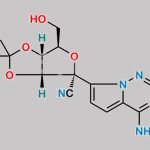
| Molecular Structure | ![Structure of Pyrrolo[1,2-f][1,2,4]triazin-4-amine CAS 159326-68-8](https://www.chemwhat.com/wp-content/uploads/2018/01/Structure-of-Pyrrolo12-f124triazin-4-amine-CAS-159326-68-8.png) |
| CAS Registry Number | 159326-68-8 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | 159326-68-8 4-aminopyrrolo<2,1-f><1,2,4>triazine, pyrrolo[2,1-f ][1,2,4]-triazin-4-amine, 4-aminopyrrolo[2,1-f][1,2,4]-triazine, pyrrolo[2,1-f][1,2,4]-triazin-4-amine, 4-aminopyrrolo[2,1-f][1,2,4]triazine, pyrrolo[2,1-f][1,2,4]triazin-4-amine, pyrrolo[2,1-f][1,2,4]triazin-4-ylamine CAS No: 159326-68-8 CAS: 159326-68-8 CAS Number: 159326-68-8 |
| Molecular Formula | C6H6N4 |
| Molecular Weight | 134.139 |
| InChI | InChI=1S/C6H6N4/c7-6-5-2-1-3-10(5)9-4-8-6/h1-4H,(H2,7,8,9) |
| InChI Key | VSPXQZSDPSOPRO-UHFFFAOYSA-N |
| Canonical SMILES | c1cc2c(ncnn2c1)N |
| Patent Information |
| No data available |
Physical Data
| Appearance | Powder |
| Solubility | No data available |
| Acidity coefficient (pKa) | 4.28±0.30(Predicted) |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 236 – 239 | H2O |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Original Text (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| 1H | d(4)-methanol | 19.84 | 1H-NMR (CD3OD): δ 7.72 (s, 1H), 7.52 (dd, 1H, J =2.5, 1.6 Hz), 6.85 (dd, 1H1 J= 4.5, 1.6 Hz), 6.64 (dd, 1 H, J= 4.5, 2.7 Hz) | Signals given | |
| 1H | d(4)-methanol | 1H-NMR (CD3OD): δ 7.72 (s, 1H), 7.52 (dd, 1H, J =2.5, 1.6 Hz), 6.85 (dd, 1H1 J= 4.5, 1.6 Hz), 6.64 (dd, 1 H, J= 4.5, 2.7 Hz) | Signals given | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6, D2O | |||
| Chemical shifts | 13C | dimethylsulfoxide-d6, D2O | |||
| Spin-spin coupling constants | 1H-1H. |
| Description (Mass Spectrometry) |
| LCMS (Liquid chromatography mass spectrometry), ESI (Electrospray ionisation) |
| LCMS (Liquid chromatography mass spectrometry), ESI (Electrospray ionisation) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | 234, 272 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In N,N-dimethyl-formamide at -20℃; for 1.5h; Experimental Procedure A stirred solution containing Intermediate A (21.0 g, 0.157 mol) in anhydrous DMF (200 ml_) was cooled to -20 0C and 1 ,3-dibromo-5,5-dimethylhydantoin (22.4 g, 0.078 mol) was added portionwise over -45 minutes. The reaction was stirred for another 45 minutes and monitored for completion by TLC (silica gel, GHLF, 5%CH3OH/CH2Cl2). Saturated25 Na2SO3 solution (300 mL) was added, the resulting suspension was stirred and the solids were collected by suction filtration. The filter cake was washed with water, dried by suction and then partitioned between ethyl acetate (1 L) and 5% sodium carbonate solution (1L). The layers were separated, the organic layer was washed with fresh sodium carbonate solution and dried over magnesium sulfate. The filtrate from the work-30 up was also extracted and combined with the main batch then filtered through a pad of Magnesol and concentrated in vacuo to afford crude mono-bromide, KRAM 206-3-1 , 29.9 g, 90% yield. Trituration of a 21.5 g quantity of the crude product in hot ethyl acetate (300 mL, 70 C) provided colorless solids (12.3 g) containing only ~2 % of the di-brominated side-product. 1H-NMR (CD3OD) : δ 7.84 (s, 1 H), 6.95 (d, 1 H, J= 4.7 Hz), 6.71 (d, 1H, J = 4.7 Hz), 4.89 (s, 3H, -NH2 + H2O); MS: LC/MS (+esi), m/z = 213.1 [M+H]. | 90% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In N,N-dimethyl-formamide at -20℃; for 1.5h; Experimental Procedure A stirred solution containing pyrrolo[2,l-f][l,2,4]triazin-4-ylamine (21.0 g, 0.157 mol) in anhydrous DMF (200 mL) was cooled to -20 0C and l,3-dibromo-5,5-dimethylhydantoin (22.4 g, 0.078 mol) was added portionwise over -45 minutes. The reaction was stirred for another 45 minutes and monitored for completion by TLC (silica gel, GHLF, 5%CH3OH/CH2C12). Saturated Na2SO3 solution (300 mL) was added, the resulting suspension was stiired and the solids were collected by suction filtration. The filter cake was washed with water, dried by suction and then partitioned between ethyl acetate (IL) and 5% sodium carbonate solution (IL). The layers were separated, the organic layer was washed with fresh sodium carbonate solution and dried over magnesium sulfate. The filtrate from the work-up was also extracted and combined with the main batch then filtered through a pad of Magnesol and concentrated in vacuo to afford crude mono-bromide, KRAM 206-3-1, 29.9 g, 90% yield. Trituration of a 21.5 g quantity of the crude mono-/di-bromo product in hot ethyl acetate (300 mL, 70 0C) provided colorless solids (12.3 g) containing only ~2 % of the di-brominated side-product. 1H-NMR (CD3OD): δ 7.84 (s, IH), 6.95 (d, IH, J = 4.7 Hz), 6.71 (d, IH, / = 4.7 Hz), 4.89 (s, 3H, -NH2 + H2O); MS: LC/MS (+esi), m/z = 213.1 [M+H]. | 90% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In N,N-dimethyl-formamide at -20℃; for 1.5h; regioselective reaction; Experimental Procedure Synthesis of 7-bromopyrrolo[2,1-f][1,2,4]triazin-4-amine: A stirred solution containing pyrrolo[2,1-f][1,2,4]-triazin-4-amine (10 g, 75 mmol) in anhydrous N,N-dimethylformamide(100 mL) was cooled to -20 °C and 1,3-dibromo-5,5-dimethylhydantoin (10.7 g, 37.4 mmol) was added portionwise over 45 min. The reaction was stirred for another45 min and monitored by TLC. After completion, the reaction mixture was washed with saturated aqueous solution of sodium sulfite (150 mL) and water. It was then partitioned between ethyl acetate (0.5 L) and 5 % aqueous solution of sodium carbonate (500 mL). The organic layer was washed with aqueous solution of sodium carbonate, dried and concentrated to afford 13.3 g crude product. It was purified by silica gel column chromatography (CH2Cl2:MeOH = 100:1) to give the7-bromo compound as a white crystal (12.3 g, 77 %). | 77% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In N,N-dimethyl-formamide at -20℃; for 1h; Experimental Procedure A Step A. 7-Bromopyrrolo[2, 1 -fill ,2,4ltriazin-4-amine. To a solution of pyrrolo[2, 1- f][l,2,4]triazin-4-amine (2.1 g, 15.66 mmol, 1.00 equiv) in DIVIF (20 mL) was added 1,3- dibromo-5,5-dimethylimidazolidine-2,4-dione (2.24 g, 7.83 mmol, 0.53 equiv) at -20 °C in batches. The resulting solution was stirred for 1 h at -20 °C, then quenched by the addition of 30 mL of sat. sodium sulfite (aq). After filtration, the filter was dissolved in 200 ml of ethyl acetate, washed with 100 mL of sat. sodium carbonate (aq.), dried over sodium sulfate and concentrated under reduced pressure. This resulted in 2.50 g (75%) of the title compound as a white solid. MS mlz [M+H]b (ESI): 213, 215. | 75% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In N,N-dimethyl-formamide at -40 – 0℃; for 0.75h; |
Safety and Hazards
| Pictogram(s) | No data available |
| Signal | No data available |
| GHS Hazard Statements | No data available [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | No data available (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | No data available |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 134.14 |
| logP | 0.97 |
| HBA | 4 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 56.21 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Effect |
| 4.06 | Kd (dissociation constant) | = | 88 | µM | Mitogen-activated protein kinase kinase kinase kinase 4 [human]:Wild | binding activity |
| 8.05 | Activity(Ligand efficiency) | = | 0.56 | Mitogen-activated protein kinase kinase kinase kinase 4 [human]:Wild | binding activity |
| Use Pattern |
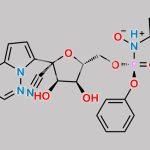
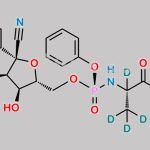
| PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE CAS#: 159326-68-8 is used as Remdesivir Intermediates. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

![Route of Synthesis (ROS) of PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE CAS 159326-68-8](https://www.chemwhat.com/wp-content/uploads/2018/01/Route-of-Synthesis-ROS-of-PYRROLO12-F124TRIAZIN-4-AMINE-CAS-159326-68-8.png)
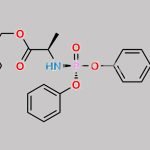
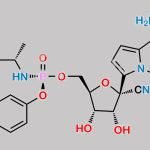
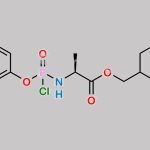
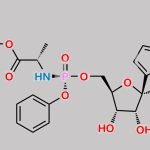
![Remdesivir 5’-Desphosphate 3’-O-[(S)phosphate] CAS#: N/A](https://www.chemwhat.com/wp-content/uploads/2024/07/remdesivir-5-desphosphate-3-o-sphosphate-150x150.jpg)