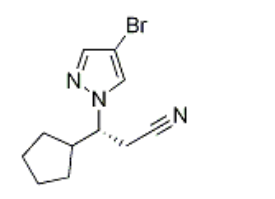
(R)-3-(4-broMo-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile CAS#: 1146629-83-5; ChemWhat Code: 1411575
Identification
Physical Data
| Appearance | Off-white solid |
| Melting Point, °C | Solvent (Melting Point) |
| 82 – 83 | cyclohexane |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Mid IR (MIR), Bands | potassium bromide |
Route of Synthesis (ROS)
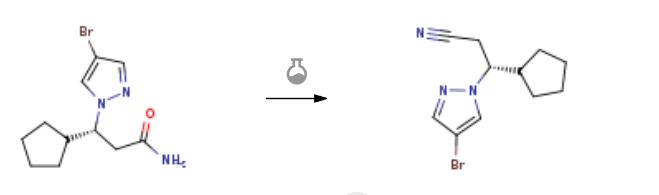
Route of Synthesis (ROS) of (R)-3-(4-broMo-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile CAS#: 1146629-83-5
| Conditions | Yield |
| With phosphorous pentoxide In tetrahydrofuran at 70℃; for 2h; Inert atmosphere; | 98% |
| With trichlorophosphate In tetrahydrofuran at 70℃; for 2h; Reagent/catalyst; Inert atmosphere; | 98% |
| With hydrogen; triethylamine In ethanol; water at 110℃; under 30003 Torr; for 24h; Autoclave; | 98% |
| With trichlorophosphate In tetrahydrofuran at 70℃; for 2h; Reagent/catalyst; Inert atmosphere; Experimental Procedure To a solution of 17 (1.24 g, 4.3 mol, 1.0 eq.), in tetrahydrofuran (50 mL), added phosphorus pentoxide (1.84 g, 13.0 mol, 3 eq.) under argon. The reaction mixture was stirred for 2 hours at 70 °C, diluted with ethyl acetate (200 mL), and quenched by adding saturated sodium bicarbonate (200 mL). The aqueous layer was back-extracted with ethyl acetate (2 x 100 mL). The organic phases were combined, washed with water, brine, dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated under reduced pressure and purified by SiO2 chromatography using methanol/dichloromethane (5 %) as eluent to provide the title compound as a white solid (1.13 g, 98%). HPLC 100% (tR = 12.56 min, CH3OH in 0.1% TFA water 5%~95% in 20 min); 1 H NMR (500 MHz, DMSO-d6) δ 8.12 (d, J= 0.5 Hz, 1H), 7.63 (d, J = 0.5 Hz, 1H), 4.38 (td, J = 9.4, 4.7 Hz, 1H), 3.15 – 3.08 (m, 2H), 2.33 – 2.28 (m, 1H), 1.79 – 1.72 (m, 1H), 1.61 – 1.38 (m, 4H), 1.30 – 1.05 (m, 3H); HPLC-MS (ESI+): m/z 268.0 (M+H)+. | 98% |
Safety and Hazards
No data available
Other Data
| Transportation | Store at room temperature, sealed and away from light |
| HS Code | |
| Storage | Store at room temperature, sealed and away from light |
| Shelf Life | 2 years |
| Market Price |
| Use Pattern |
| (R)-3-(4-broMo-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile CAS#: 1146629-83-5 is used in pharmaceutical intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |