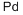Ruthenium CAS#: 7440-18-8; ChemWhat Code: 17721
Identification
| Product Name | Ruthenium |
| IUPAC Name | ruthenium |
| Molecular Structure | |
| CAS Registry Number | 7440-18-8 |
| EINECS Number | 231-127-1 |
| MDL Number | MFCD00011207 |
| Synonyms | ruthenium, rhuthenium, ruthenium,, Rutenium, Ru, Rh, Ruthenium; CAS Number 7440-18-8; CAS NO 7440-18-8 |
| Molecular Formula | [Ru] |
| Molecular Weight | 101.07 |
| InChI | InChI=1S/Ru |
| InChI Key | KJTLSVCANCCWHF-UHFFFAOYSA-N |
| Canonical SMILES | [Ru] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/121400 | PROMOTED RUTHENIUM CATALYST FOR THE IMPROVED HYDROGENATION OF CARBOXYLIC ACIDS TO THE CORRESPONDING ALCOHOLS | 2014 |
| WO2009/53446 | NOVEL PREGABALIN INTERMEDIATES AND PROCESS FOR PREPARING THEM AND PREGABALIN | 2009 |
| WO2008/69984 | PRODUCTION OF BUTENES AND DERIVATIVES THEREFORM FROM DRY ETHANOL | 2008 |
Physical Data
| Appearance | Wet black powder |
| Melting Point | 2310 °C (lit.) |
| Boiling Point | 3900 °C(lit.) |
| Flash Point | 207.324°C |
| Density | 12.45 g/ml |
| Description (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Adsorption to title compound | CO and H |
| Adsorption to title compound | carbon monoxide |
| Adsorption to title compound | methanol |
| Adsorption to title compound | CO |
| Adsorption to title compound | chlorine |
Spectra
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Spectrum | neat (no solvent, solid phase) | 1000 cm**-1 – 3000 cm**-1 | |
| Spectrum | neat (no solvent) | 256.85 | 1900 cm**-1 – 2200 cm**-1 |
| Spectrum | neat (no solvent, solid phase) | 2800 cm**-1 – 4000 cm**-1 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum, Band assignment | ethane-1,2-diol | 200 nm – 800 nm |
| Spectrum, Band assignment | solid | 0.5 eV – 6.5 eV |
| Spectrum, Band assignment | neat (no solvent) | 260 nm – 380 nm |
Route of Synthesis (ROS)

| Conditions | Yield |
| With carbon monoxide In neat (no solvent) passing CO over RuO2, heating; | |
| In neat (no solvent) heating at 1400 °C, cooling; | |
| With hydrogen at 200℃; for 4h; α-aluminium oxide; Experimental Procedure 300 g of a used catalyst comprising 2percent by weight of RuO2 on α-aluminum oxide as support was reduced in a stream of hydrogen at 200° C. in a rotary tube furnace for 3 hours. Here, the catalyst was firstly heated to 200° C. in a stream of nitrogen, and was then reduced in a stream of N2/H2 (5:1) for 1 hour and subsequently in pure H2 for 3 hours. The reduced catalyst was dispensed under a nitrogen atmosphere into a vessel into which nitrogen was passed. The reduced catalyst was subsequently transferred under a nitrogen atmosphere into a 2000 ml stirred apparatus and 1160 g of 32percent strength by weight hydrochloric acid were added. While stirring and introducing nitrogen, the mixture was heated to 100° C. The mixture was subsequently stirred at 100° C. while sparging the solution with 50 l/h of air for 24 hours. A dark red ruthenium chloride solution was obtained and this was decanted off from the residue. The support which remained as residue was washed with about 1 l of water until neutral. The support was subsequently dried at 120° C. for 16 hours and the ruthenium content of the support was determined. The determination indicated a ruthenium content of 0.20percent by weight. In comparison, the used catalyst comprised 1.49percent by weight of ruthenium. Thus, about 13percent of the originally comprised ruthenium remained on the support. | |
| With CO In neat (no solvent) passing CO over RuO2, heating; | |
| In neat (no solvent) heating at 1400 °C, cooling;; | |
| With H2 In neat (no solvent) reduction of RuO2 in hydrogen at 873 K within 2 h; | |
| With H2 In neat (no solvent) reduction of RuO2 (commercial compound or prepared from RuCl3 by calcining in air) in flowing H2 (1.7 cm**3/s) at temps. between 553 and 803 K; degassing at react. temp. for 3 h down to 1.33E-4 Pa; | |
| In neat (no solvent) RuO2 decompd. into metal at temp. of about 100°C at heating rate 1°C/min under Ar/H2; detn. by TG anal.; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H228: Flammable solid [Danger Flammable solids] H413: May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P210, P240, P241, P273, P280, P370+P378, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 381512 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 790/kg |
| Use Pattern |
| catalyst for producing monofunctionalized dialkylphosphinic acids, esters or salts |
| manufacturing a hydrocarbon |
| enantioselective preparation of herbicide metolachlor precursor (S)-2-ethyl-N-(1-methoxypropan-2-yl)-6-methylaniline |
| Ruthenium is mostly used in effective hardener |
| catalyst for fluoroolefins hydrogenation |
| metallic element for hydrodenitrogenation catalyst |
| Catalyst useful for N-demethylation and/or N-acylation of N-methylated heterocycle |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |