Selenium sulfide CAS#: 7488-56-4; ChemWhat Code: 122890
Identification
Physical Data
| Appearance | Red powder |
Spectra
| Description (IR Spectroscopy) |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
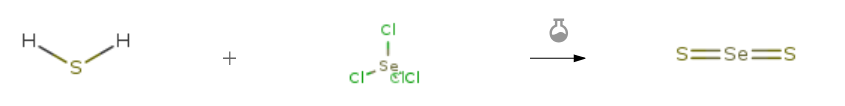
| In further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide;; | |
| In further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide; | |
| In further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide; |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H300 (10.09%): Fatal if swallowed [Danger Acute toxicity, oral] H301+H331 (33.94%): Toxic if swallowed or if inhaled [Danger Acute toxicity, oral; acute toxicity, inhalation] H301 (86.24%): Toxic if swallowed [Danger Acute toxicity, oral] H330 (11.93%): Fatal if inhaled [Danger Acute toxicity, inhalation] H331 (84.4%): Toxic if inhaled [Danger Acute toxicity, inhalation] H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H400 (94.5%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (93.58%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P260, P261, P264, P270, P271, P273, P284, P301+P316, P304+P340, P316, P319, P320, P321, P330, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 143.092 |
| logP | -0.296 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 64.18 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Selenium disulfide, also known as selenium sulfide, is a chemical compound with the formula SeS2, where selenium and sulfur are combined in a 1:2 ratio. It is best known for its application in medical and personal care products, particularly as an active ingredient in anti-dandruff shampoos and as a treatment for certain skin conditions. TSelenium disulfide’s unique properties and its role in health and skincare highlight its importance in both medical dermatology and cosmetic formulations. The primary mechanism through which selenium disulfide exerts its effects is by reducing the proliferation of Malassezia, a genus of fungi that naturally resides on the skin. Overgrowth of these fungi can lead to dandruff and seborrheic dermatitis, conditions characterized by flaky and itchy scalp. Selenium disulfide’s antifungal properties help regulate the presence of these fungi, thereby controlling the symptoms associated with their overgrowth. Apart from its antifungal action, Selenium disulfide is also known for its keratolytic activity, meaning it can help in removing dead skin cells and reducing scaliness. This makes it effective not only against dandruff but also in the treatment of tinea versicolor, a fungal infection that affects the skin’s pigmentation, resulting in discolored patches. In the formulation of anti-dandruff shampoos and lotions, the concentration of selenium disulfide is carefully regulated. Typically, over-the-counter products contain it at concentrations ranging from 1% to 2.5%, ensuring effectiveness while minimizing potential side effects. These may include skin irritation, oiliness or dryness of the scalp and hair, and a temporary change in hair color. Despite these side effects, selenium disulfide is considered safe for most users when used as directed. The use of selenium disulfide extends beyond personal care products; it also has implications in various industrial applications. However, its most significant impact remains in the field of dermatology, where it provides a critical solution for managing dandruff, seborrheic dermatitis, and tinea versicolor. Ongoing research into selenium disulfide aims to further understand its mechanisms of action, potential resistance development by target fungi, and ways to enhance its efficacy and reduce side effects. As science advances, new formulations and applications of selenium disulfide may emerge, expanding its role in healthcare and personal care industries. In summary, selenium disulfide is a vital compound in the fight against dandruff and certain skin conditions, offering relief to millions of people worldwide. Its continued study and application promise to improve the quality of life for those affected by these common yet troublesome conditions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |