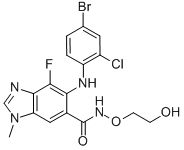
Selumetinib CAS#: 606143-52-6; ChemWhat Code: 41518
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109438362 | Substituted benzimidazole compound and composition with compound | 2019 |
| WO2018/65924 | INTERMEDIATES OF MITOGEN-ACTIVATED PROTEIN KINASE KINASE (MAP2K OR MEK) INHIBITORS AND PROCESS FOR THEIR PREPARATION | 2018 |
| US2004/116710 | N3 alkylated benzimidazole derivatives as MEK inhibitors | 2004 |
| US2003/232869 | N3 alkylated benzimidazole derivatives as MEK inhibitors | 2003 |
Physical Data
| Appearance | White or off-white powder. |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Solid state NMR, Chemical shifts, Spectrum | 19F | 376 | |
| 1H | CD3OD | 400 | |
| 19F | CD3OD | 376 | |
| 1H | CD3OD |
Route of Synthesis (ROS)
| Conditions | Yield |
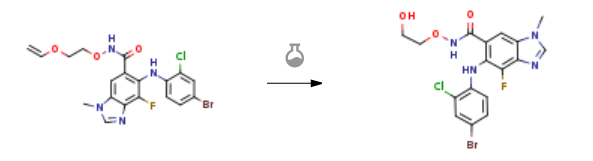
| With hydrogenchloride; water In ethanol for 24h; Experimental Procedure Hydrochloric acid (14 mL, 1.0 M aqueous solution, 14 mmol) was added to a suspension of 6-(4-bromo-2-chloro- phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboyxlic acid (2-vinyloxyethoxy)- amide (2.18 g, 4.50 mmol) in ethanol (50 mL) and the reaction mixture allowed to stir for 24 hours. The reaction mixture was concentrated to dryness by rotary evaporation and the solids partitioned between 3:1 ethyl acetate/tetrahydrofuran and saturated potassium carbonate. The aqueous phase was extracted with 3:1 ethyl acetate/tetrahydrofuran (3x), the combined organics dried (Na2SO4), and concentrated to provide 2.11 g (100%) 6-(4-bromo-2- chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2- hydroxyethoxy)-amide as an off-white solid. MS ESI (+) m/z 457, 459 (M+, Br pattern) detected. 1H NMR (400 MHz, MeOH-&0 δ 8.26 (s, IH), 7.78 (s, IH), 7.57 (d, IH), 7.24 (dd, IH), 6.40 (dd, IH), 3.86 (s, 3H), 3.79 (m, 2H), 3.49 (m, 2H). 19F NMR (376 MHz, MeOH-d4) -133.68 (s). | 100% |
| With hydrogenchloride In ethanol; water at 25 – 30℃; for 24h; Experimental Procedure 7 Example 7: Preparation of 5-[(4-Bromo-2-chlorophenyl)aminoj-4-fluoro-N-(2- hydroxyethoxy)- 1-methyl- 1H-benzimidazole-6-carboxamide (Selumetinib) Aqueous hydrochloric acid (iN, 1.2 mL hydrochloric acid in 12 mL deionized water) was added slowly to a solution of 5-[(4-Bromo-2-chlorophenyl)aminoj-N-[2-(ethenyloxy)ethoxyj -4-fluoro- 1-methyl- 1H-benzimidazole-6-carboxamide (1.3 g; obtained from Example 6) in ethanol (30 mL) over a period of 10 minutes and the reaction mixture was stirred at 25°C to 30°C for 24 hours. After completion of the reaction (monitored by TLC), ethanol was concentrated under vacuum. Ethyl acetate: tetrahydrofuran (3:1, 45:15 mL) was added and the organic layer was washed with saturated potassium carbonatesolution (25 mL). The organic layer was separated and the aqueous layer was washed with ethyl acetate: tetrahydrofuran (45: 15 mL) solution. The organic layers were combined and dried over sodium sulphate and then concentrated under vacuum to obtain a crude compound as a brown solid (950 mg). The crude compound was further purified by column chromatography using 2% methanol/dichloromethane as an eluent to obtain thetitle compound.Yield: 70% | 70% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H411 (100%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P260, P261, P264+P265, P272, P273, P280, P302+P352, P305+P354+P338, P317, P318, P319, P321, P333+P317, P362+P364, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 457.687 |
| logP | 3.696 |
| HBA | 5 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 88.41 |
| Rotatable Bond (RotB) | 7 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Selumetinib CAS#: 606143-52-6 is used for the treatment of advanced non-small cell lung cancer (NSCLC). Selumetinib primarily inhibits the growth of various tumors, including melanomas with B-Raf mutations and non-small cell lung cancer (NSCLC) with K-Ras mutations, by regulating the key protein kinase MEK in the Ras-Raf-MEK-ERK pathway. It is mainly employed in the treatment of diseases such as cholangiocarcinoma, colorectal cancer, and NSCLC. Currently, Selumetinib is in phase III clinical trials for the treatment of non-small cell lung cancer. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |