SODIUM METHACRYLATE CAS#: 5536-61-8; ChemWhat Code: 35679
Identification
| Product Name | SODIUM METHACRYLATE |
| IUPAC Name | sodium;2-methylprop-2-enoate |
| Molecular Structure | 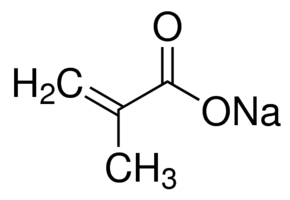 |
| CAS Registry Number | 5536-61-8 |
| EINECS Number | 226-896-5 |
| MDL Number | MFCD00045886 |
| Synonyms | sodium methacrylate, methacrylic acid sodium salt, sodium methylmethacrylate, sodium 2-methacrylate, Sodium methacrylate, sodium metacrylate, SMA |
| Molecular Formula | C4H5NaO2 |
| Molecular Weight | 108.07 |
| InChI | InChI=1S/C4H6O2.Na/c1-3(2)4(5)6;/h1H2,2H3,(H,5,6);/q;+1/p-1 |
| InChI Key | SONHXMAHPHADTF-UHFFFAOYSA-M |
| Canonical SMILES | CC(=C)C(=O)[O-].[Na+] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2016/196434 | (Meth) acrylate-containing halogenated phosphate ester mixture and its manufacturing method, and a cured composition comprising a mixture of (by machine translation) | 2016 |
| EP2100887 | Lactone-containing compound, polymer, resist composition, and patterning process | 2009 |
| US6346650 | Method for making mixed high purity (meth)acrylic anhydrides | 2002 |
Physical Data
| Appearance | White superfine powder |
| Water Solubility | Soluble in water. |
| Metling Point | 300°C |
| Boiling Point | 100 °C |
| Density | 2.703g/cm3@25°C |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | ethanol-d6 | 40 | 500.16 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| SODIUM METHACRYLATE CAS 5536-61-8 IR | 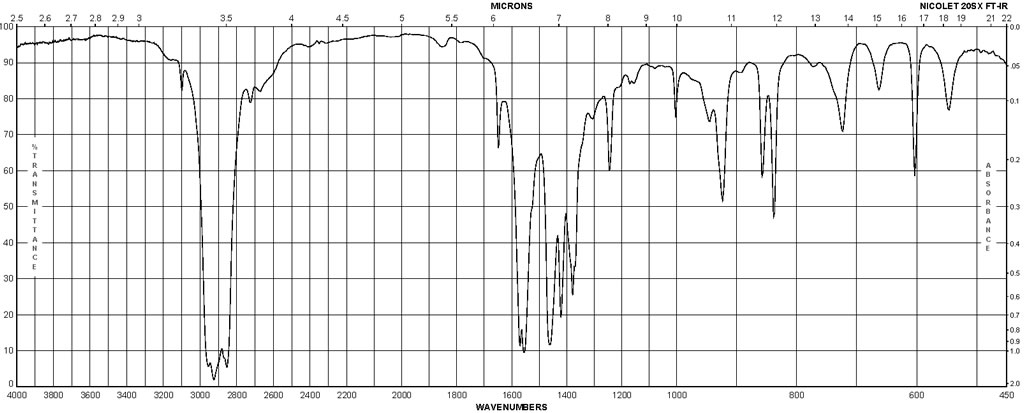 |
Route of Synthesis (ROS)
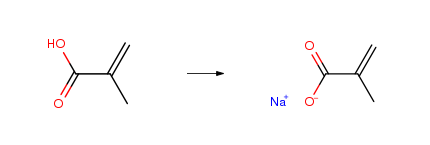
| Conditions | Yield |
| With sodium hydroxide In water; acetone Experimental Procedure Preparation of Sodium Methacrylate A 12.23 g (0.3058 mol) sample of sodium hydroxide was placed in a 50-ml Erlenmayer flask equipped with 10 ml water and a magnetic stirring bar, and the mixture was stirred to dissolution. The sodium hydroxide solution was then poured into a 250-ml beaker containing 30.70 g (0.3570 mol) methacrylic acid (exothermic reaction) with constant stirring. The mixture was allowed to cool, 50 ml of acetone were added, and the precipitate vacuum filtered. The recovered carboxylate salt was first allowed to air dry under a hood, and then dried in an oven at 55°-60° C. for 12-15 hours to afford 30.43 g (92.2percent yield) of sodium methacrylate. | 92.2% |
| With sodium hydroxide Experimental Procedure Preparation of sodium methacrylate: After 5 kg (37.5 mol) of a 30percent aqueous solution of sodium hydroxide was cooled below 60° C., 3.23 kg (37.5 mol) of methacrylic acid was added drop by drop while stirring. The aqueous solution of sodium methacrylate obtained by the neutralization reaction was spray-dried with 300° C. hot air to yield 4.04 kg of a dry powder of sodium methacrylate. The water content of this powder was 0.08percent. | |
| With sodium hydroxide In water at 20 – 50℃; for 2.5h; Product distribution / selectivity; Experimental Procedure Example 3; A. Preparation of Sodium Methacrylate Solution; To a five liter 4-neck round bottom flask fitted with a reflux condenser, thermocouple, and mechanical stirrer was charged 1500.11 grams of de-ionized water. To this was incrementally added a total of 511.63 grams (12.79 moles) of sodium hydroxide pellets. The addition rate was such that the pot temperature did not exceed 50° C. After the solution cooled to room temperature, 1115.40 grams (12.82 moles) of methacrylic acid was added over the course of 2.5 hours. The temperature of the reaction mixture was kept below 50° C. throughout the methacrylic acid addition. This preparation resulted in a clear, colorless solution containing 44.1percent by weight sodium methacrylate. | |
| With sodium methylate In methanol for 1.3h; Product distribution / selectivity; Experimental Procedure Example 5; This example illustrates the use of sodium methoxide as the neutralizing base. To a 500 ml 4-neck round bottom flask equipped with a reflux condenser, addition funnel, thermocouple and magnetic stirrer was charged 38.67 grams (0.45 moles) of methacrylic acid and 52.12 grams of methanol. To the addition funnel was charged 95.89 grams of a 25percent solution of sodium methoxide in methanol (23.97 grams of contained sodium methoxide, 0.44 moles, 98.6percent of theoretical). The sodium methoxide solution was slowly added to the methacrylic acid over a 1.3 hour period with vigorous stirring. Near the end of the addition the amount of formed solids made stirring difficult. After all of the sodium methoxide solution was charged, the addition funnel was rinsed with 17.53 grams of methanol. To the resulting thick slurry was added 3.21 grams of a 34percent aqueous solution of hexaethylguanidinium chloride solution (1.09 grams of contained hexaethylguanidinium chloride, 4.1 mmoles). This material was rinsed into the reaction flask with 7.58 grams of methanol. The methanol was removed by mildly heating the reaction slurry under vacuum. The remaining paste was further dried by heating in a vacuum oven at 90° C. and 125 mmHg overnight. The resulting dry solids were broken up with a spatula and the reaction flask fitted with a reflux condenser, thermocouple and mechanical stirrer. To the solids were added 0.192 grams (0.45 mmoles) of 4,4′-methylenebis(2,6-di-t-butylphenol), 0.081 grams (0.36mmoles) of butylated hydroxytoluene (BHT) and 131.58 grams (0.66 moles) of chloropropyltrimethoxysilane. The mixture was then heated to 110° C. for 3 hours. During this time the color of the reaction mixture changes from purple to yellow. Analysis of the final product by gas chromatograpy indicated that the crude reaction mixture contained 72.3percent of the desired gamma-methacryloxypropyltrimethoxysilane. |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H315: Causes skin irritation [Warning Skin corrosion/irritation] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | No data valiable |
| Use Pattern |
| SODIUM METHACRYLATE CAS#: 5536-61-8 can be used as dispersing agent; |
| SODIUM METHACRYLATE CAS#: 5536-61-8 can be used as coating materials. |
| Sodium Methacrylate polymerization reaction to poly acrylic acid |
| SODIUM METHACRYLATE CAS#: 5536-61-8 is a kind of poly anionic polymer dielectric, is a widely used and important chemicals. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
