t-BuXPhos CAS#: 564483-19-8; ChemWhat Code: 38269
Identification
| Product Name | t-BuXPhos |
| IUPAC Name | ditert-butyl-[2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane |
| Molecular Structure | 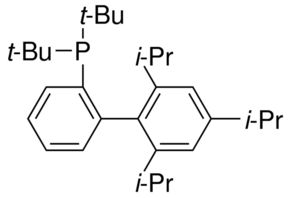 |
| CAS Registry Number | 564483-19-8 |
| MDL Number | MFCD06411306 |
| Synonyms | tert-butyl XPhos, di-t-butyl(2′,4′,6′-triisopropyl-3,4,5,6-tetramethyl-(1,1′-biphenyl)-2-yl)phosphine, 2-di-tert-butyl(2’,4‘,6‘-triisopropyl-[1,1‘-biphenyl]-2-yl)phosphine, di-tert-butyl(2’,4’,6’-triisopropyl-[1,1‘-biphenyl]-2-yl)phosphine, di-tert-butyl(2’,4’,6’-triisopropyl-[1,1’-biphenyl]-2-yl)phosphine, di-tert-butyl(2’,4’,6’-triisopropyl[1,1‘-biphenyl]-2-yl)phosphine, di-tertbutyl (2’,4’,6’-triisopropyl[1,1‘-biphenyl]-2-yl)phosphine;CAS Number: 564483-19-8 |
| Molecular Formula | C29H45P |
| Molecular Weight | 424.64 |
| InChI | InChI=1S/C29H45P/c1-19(2)22-17-24(20(3)4)27(25(18-22)21(5)6)23-15-13-14-16-26(23)30(28(7,8)9)29(10,11)12/h13-21H,1-12H3 |
| InChI Key | SACNIGZYDTUHKB-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)c1cc(c(c(c1)C(C)C)c2ccccc2P(C(C)(C)C)C(C)(C)C)C(C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN105859774 | A preparation method of compound phosphine benzene apperception (by machine translation) | 2016 |
| US2013/23678 | PROCESS FOR PREPARING SULFONAMIDOBENZOFURAN DERIVATIVES | 2013 |
| US2018/230157 | CATALYST AND PROCESS FOR THE CO-DIMERIZATION OF ETHYLENE AND PROPYLENE | 2015 |
| US2011/15401 | Metal-Catalyzed Carbon-Fluorine Bond Formation | 2011 |
| EP2062901 | FLUOROBORON COMPOUND HAVING AROMATIC RING OR SALT THEREOF, AND PROCESS FOR PRODUCTION OF COMPOUND HAVING CYCLIC ETHER-FUSED AROMATIC RING BY USING THE SAME | 2009 |
Physical Data
| Appearance | White crystal powder |
| Boiling Point | 493.5±45.0 °C(Predicted) |
| Melting Point, °C |
| 144~145 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | benzene-d6 | 300 | ||
| Chemical shifts | 13C | benzene-d6 | 121 | ||
| Chemical shifts | 31P | ||||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 400 | |
| Chemical shifts, Spectrum | 31P | chloroform-d1 | 25 | 162 | |
| 1H | 1H | benzene-d6 | 300 | ||
| Chemical shifts | 31P | benzene-d6 | 121 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | solid |
Route of Synthesis (ROS)
| Conditions | Yield |
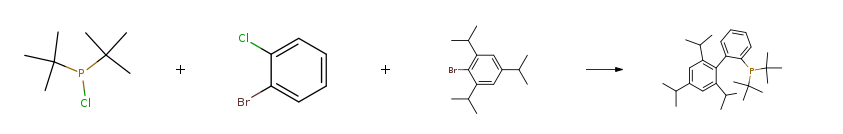
| Stage #1: 2-bromo-1-chlorobenzene; 2,4,6-triisopropyl-1-bromobenzene With magnesium In tetrahydrofuran for 2h; Inert atmosphere; Reflux; Stage #2: di(tert-butyl)chlorophosphine With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 20℃; for 5.5h; Reagent/catalyst; Reflux; Inert atmosphere; Experimental Procedure 5 Example 5 Preparation of 2′-di -tert-butylphosphino-2,4,6-triisopropylbiphenyl 1L three bottles,To a solution of 32.5 g of 2,4,6-triisopropylbromobenzene,5.6 g of magnesium turnings and 300 mL of anhydrous THF,20 g of o-chlorobromobenzene was dropwise added,2,4,6-triisopropyl-2-bromobiphenyl Grignard reagent,Refluxed for 2 hours,Down to room temperature,2.4 g of tetrakis (triphenylphosphine) palladium was added,Stirred for 30 minutes,18.8 g of di-tert-butylphosphonium chloride was added dropwise at room temperature,The reaction was refluxed for 5 hours.And the mixture was added dropwise to the reaction solution under ice-water bath200 mL of saturated aqueous ammonium chloride was quenched,Liquid separation,The organic phase is dissolved,Add methanol crystallization,And filtered to obtain 41.7 g of white 2-di-tert-butylphosphine-2,4,6-triisopropylbiphenyl,Yield 94%. | 94% |
| Stage #1: 2,4,6-triisopropyl-1-bromobenzene With magnesium; ethylene dibromide In tetrahydrofuran at 65℃; for 1h; Stage #2: 2-bromo-1-chlorobenzene In tetrahydrofuran at 65℃; for 1h; Stage #3: di(tert-butyl)chlorophosphine With copper(l) chloride In tetrahydrofuran at 20℃; for 20h; | 32% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (98.59%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (98.59%): Harmful in contact with skin [Warning Acute toxicity, dermal] H332 (98.59%): Harmful if inhaled [Warning Acute toxicity, inhalation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293100 |
| Storage | Under the room temperature and away from light |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 424.65 |
| logP | 11.292 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 13.59 |
| Rotatable Bond (RotB) | 7 |
| Matching Veber Rules | 2 |
| Bioactivity |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 3.82 | inhibition rate(of cell proliferation) | < | 50 | % | antiproliferative agent |
| Use Pattern |
| Palladium-catalyzed Tsuji-Trost substitution and cross-coupling of benzyl fluoride. |
| Palladium-catalyzed C-N cross-coupling of sulfinamide and aryl halide. |
| Palladium-catalyzed rapid methoxylation and deuteration of bromo-bromochalcone. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |