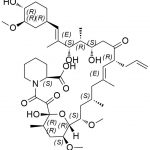
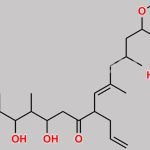
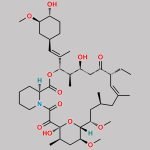
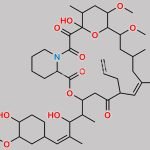
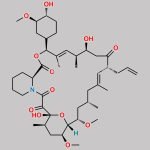
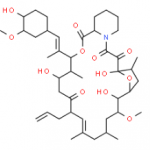
Tacrolimus Regioisomer CAS#: 131944-48-4; ChemWhat Code: 1421672
Identification
| Product Name | Tacrolimus Regioisomer |
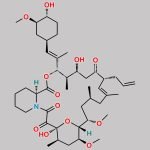
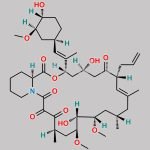
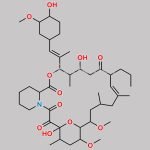
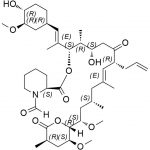
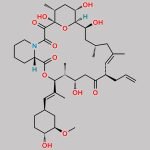
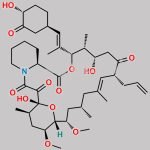
| Molecular Structure | 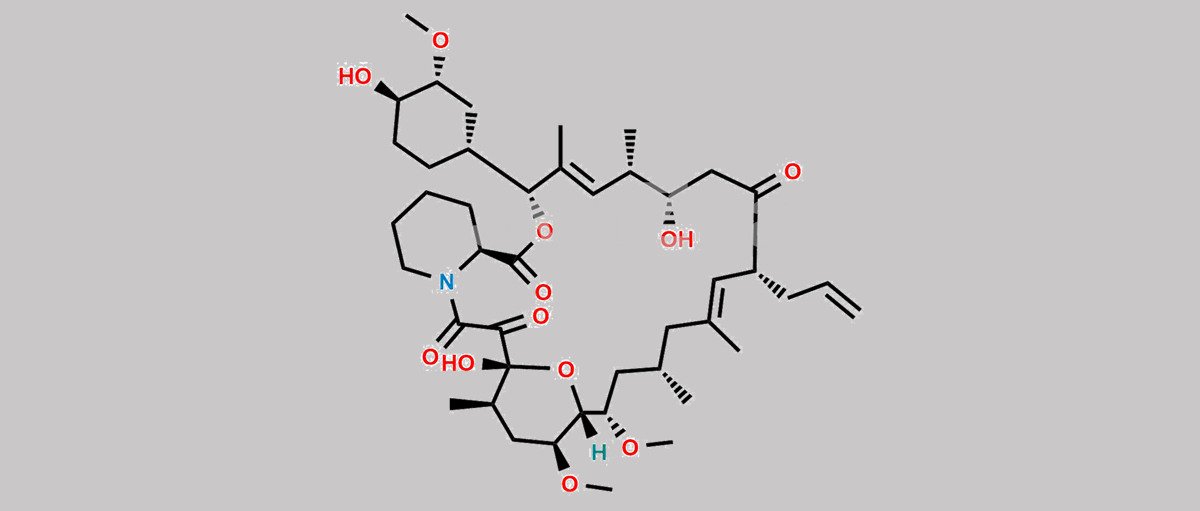 |
| CAS Registry Number | 131944-48-4 |
| EINECS Number | |
| MDL Number | |
| Beilstein Registry Number | |
| Synonyms | 17,21-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclopentacosine-1,9,22,23(6H,25H)-tetrone, 7,8,10,13,14,15,16,17,18,19,20,21,26,27,28,28a-hexadecahydro-7,21-dihydroxy-3-(4-hydroxy-3-methoxycyclohexyl)-16,18-dimethoxy-4,6,12,14,20-pentamethyl-10-(2-propenyl)-, [3R-[3R*(1R*,3R*,4R*),4E,6S*,7S*,10R*,11E,14S*,16S*,17R*,18S*,20R*,21R*,28aS*]]- (9CI) |
| Molecular Formula | C44H69NO12 |
| Molecular Weight | 804.0 |
| InChI | |
| InChI Key | |
| Canonical SMILES | O[C@H]1[C@H](OC)C[C@H]([C@H](/C(C)=C/[C@H](C)[C@H](CC2=O)O)OC([C@H]3N(C(C([C@@]4(O)[C@H](C)C[C@H](OC)[C@@]([H])([C@@H](OC)C[C@@H](C)C/C(C)=C/[C@H]2CC=C)O4)=O)=O)CCCC3)=O)CC1 |
Physical Data
| Appearance | No data available |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point | No data available |
Spectra
| No data available |
Route of Synthesis (ROS)
| No data available |
Safety and Hazards
| No data available |
Other Data
| HS Code | 382290 |
| Storage | At room temperature and away from light |
| Related Chemicals |
| Use Pattern |
| Tacrolimus Regioisomer is chemically 17,21-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclopentacosine-1,9,22,23(6H,25H)-tetrone, 7,8,10,13,14,15,16,17,18,19,20,21,26,27,28,28a-hexadecahydro-7,21-dihydroxy-3-(4-hydroxy-3-methoxycyclohexyl)-16,18-dimethoxy-4,6,12,14,20-pentamethyl-10-(2-propenyl)-, [3R-[3R*(1R*,3R*,4R*),4E,6S*,7S*,10R*,11E,14S*,16S*,17R*,18S*,20R*,21R*,28aS*]]- (9CI). Tacrolimus Regioisomer is supplied with detailed characterization data compliant with regulatory guideline. Tacrolimus Regioisomer can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Tacrolimus. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ChemWhat products are for analytical purpose only and not for human use. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |