(-)-Taddol CAS#: 93379-48-7; ChemWhat Code: 65056
Identification
| Product Name | (-)-Taddol |
| IUPAC Name | [(4R,5R)-5-[hydroxy(diphenyl)methyl]-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanol |
| Molecular Structure | 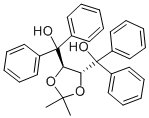 |
| CAS Registry Number | 93379-48-7 |
| Beilstein Registry Number | 3657855 |
| Synonyms | (R,R)-TADDOL, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-(-)-2,2-dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4R,5R)-2,2-Dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxolane-4,5-dimethanol, α,α,α’,α’-tetraphenyl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)-dimethanol, (R,R)-α,α,α’,α’-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol |
| Molecular Formula | C31H30O4 |
| Molecular Weight | 466.568 |
| InChI | InChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m1/s1 |
| InChI Key | OWVIRVJQDVCGQX-VSGBNLITSA-N |
| Canonical SMILES | CC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C |
| Isomeric SMILES | CC1(O[C@H]([C@@H](O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN104844654 | A quaternary phosphonium salt compound and its preparation method (by machine translation) | 2016 |
| US2009/30235 | METHOD FOR FRACTIONATING STEREOISOMERIC COMPOUNDS | 2009 |
| US6184404 | Process for the selective alkylation of aldehydes by means of organozinc compounds | 2001 |
Physical Data
| Appearance | White solid |
| Melting Point, °C | Solvent (Melting Point) |
| 196 – 197 | |
| 192 – 194 | |
| 185 | |
| 211 – 212 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.213 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| NMR spectrum of the complex | CDCl3 | 25 | 3,3′-dimethoxy-2,2′-bipyridine-N,N’-dioxide |
| Association with compound | 25 | (S)-1-phenylethanol | |
| Association with compound | 2-oxo-2-phenyl-N,N-dipropylacetamide | ||
| Association with compound | N,N-diethyl-2-oxo-2-phenylacetamide | ||
| NMR spectrum of the complex | CDCl3 | 2-Amino-3-methyl-pentanoic acid methyl ester | |
| NMR spectrum of the complex | CDCl3 | (R)-isopropyl 2-aminopropanoate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 200 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts | 13C | chloroform-d1 | 75 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
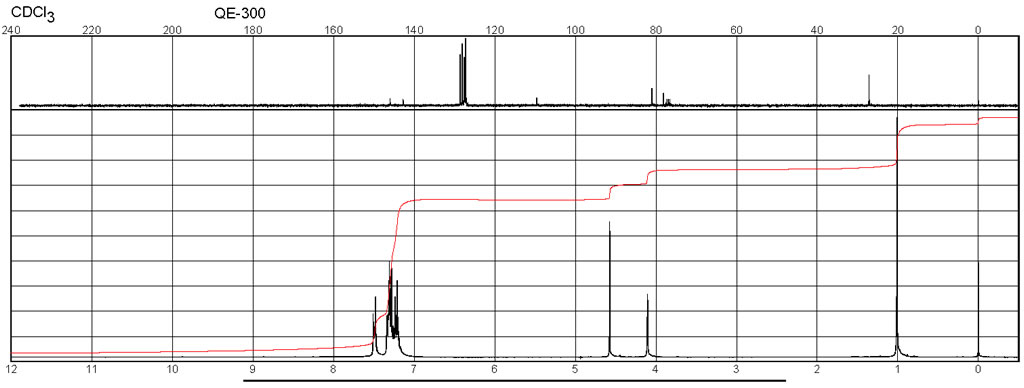
| Spectrum | 1H | CDCl3 | 400.13 |
| Spectrum | 13C | CDCl3 | 100.613 |
| Chemical shifts | 1H | CDCl3 | 200 |
| Chemical shifts | 1H | CDCl3 | 300 |
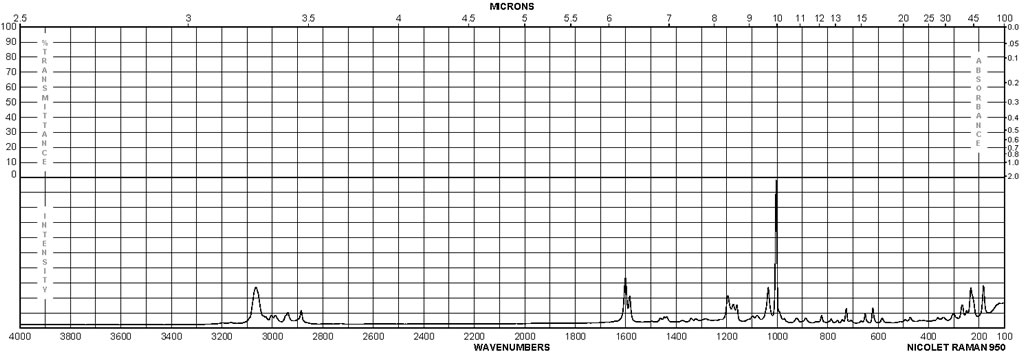
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands | ||
| Bands, Spectrum | neat (no solvent, solid phase) | |
| Spectrum | CH2Cl2 | |
| Bands | KBr | |
| Bands | 3434 – 3206 cm**(-1) | |
| Bands | 3600 – 3400 cm**(-1) | |
| Bands | CHCl3 | 3590 – 1815 cm**(-1) |
| Description (UV/VIS Spectroscopy)nm | Solvent (UV/VIS Spectroscopy) | Ext./Abs. Coefficient, l·mol-1cm-1 |
| 193 | 134000 |
Route of Synthesis (ROS)
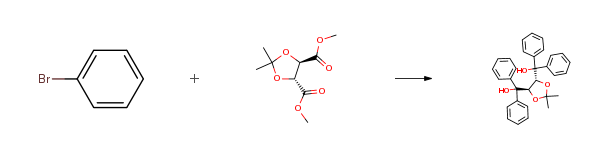
| Conditions | Yield |
| Stage #1: bromobenzene With n-butyllithium In diethyl ether; hexane at 20℃; for 2h; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In diethyl ether; hexane at 20℃; | 92% |
| Stage #1: bromobenzene With magnesium In tetrahydrofuran Cooling with ice; Inert atmosphere; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux; | 91% |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Reflux; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran Inert atmosphere; Cooling with ice; Stage #3: In tetrahydrofuran for 1.5h; Inert atmosphere; Reflux; | 88% |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran for 1h; Reflux; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 1.5h; Reflux; Experimental Procedure Under argon atmosphere, a reflux vessel was installed on a three-necked flask,A drip funnel with a pressure balance valve, and a thermometer; then add fresh magnesium bars 4.1 g, 579 mmol, 1.02 equiv.) And a small piece of iodine as the initiator. Then, bromobenzene (86.5 g, 551 mmol) was added to the dropping funnel,In tetrahydrofuran (386 mL) was added dropwise slowly until the reaction started. Constantly dropping to the end,The reaction was continued by reflux for one hour and then cooled to room temperature.To the above-mentioned format reagent, dimethyl tartrate 2 (24.1 g, 124 mmol) was added, slowly added,To ensure that the temperature does not exceed 20 degrees, after the drop is completed,The reaction system was heated to reflux for 1.5 hours,And then cooled to room temperature.Slowly adding saturated ammonium chloride solution quenching reaction,Extracted three times with ethyl acetate (40 mL X3)Then dried over anhydrous magnesium sulfate; filtered, dried and dried in vacuo to give a slightly yellow foamy solid;Recrystallization from methylene chloride and methanol gave white solid 4 (50.8 g, 88percent yield). | 88% |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran Inert atmosphere; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran for 8h; Inert atmosphere; Experimental Procedure 705 g (290 mmol) of metal magnesium was ground and ground, and then poured into 90 mL of dry treated anhydrous tetrahydrofasmonan,A small pellet was added and 42.06 g (267.9 mmol) of bromobenzene was dissolved in 120 mL of anhydrous tetrahydrofenamyl,In the N2 protection, the first small amount of drop into the magnesium iodine mixture, to be yellow solution faded, there are bubbles emerge, and then continue to drop the remaining bromobenzene tetrahydrofuran solution, such as magnesium dissolved disappear, the reaction was gray-green, Then 9.77 g (44.8 mmol) of the ketal-protected dimethyl tartrate was dissolved in 90 mL of anhydrous tetrahydrofuran and added dropwise to the format reagent under an oil bath for about 8 h,The reaction was quenched with saturated aqueous ammonium chloride solution, The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were washed twice with saturated brine, dried over anhydrous magnesium sulfate and recrystallized from methanol to give the product as a white solid Α, α, α ‘, α-tetraphenyl-1,3-dioxolane-4,5-dimethanol 16 · 28 g, yield 78percent. | 78% |
| Stage #1: bromobenzene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Reflux; Stage #2: (-)-dimethy-2,3-O-isopropylidene-L-tartrate In tetrahydrofuran at 0℃; for 1.5h; Inert atmosphere; Reflux; | 62% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| (-)-Taddol CAS#: 93379-48-7 is used as a chiral ligand for enantioselective oxidative coupling of 3-phenylacetyl-2-oxazolidinone to afford dimer with good enantioselectivity |
| phase transfer catalyst for Schiff’s base alkylation |
| Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2 |
| Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions |
| Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |