(+)-Taddol CAS#: 93379-49-8; ChemWhat Code: 65018
Identification
| Product Name | (+)-Taddol |
| IUPAC Name | [(4S,5S)-5-[hydroxy(diphenyl)methyl]-2,2-dimethyl-1,3-dioxolan-4-yl]-diphenylmethanol |
| Molecular Structure | 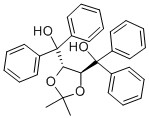 |
| CAS Registry Number | 93379-49-8 |
| Beilstein Registry Number | 4724097 |
| MDL Number | MFCD00010079 |
| Synonyms | (S,S)-taddol, (4S,trans)-2,2-dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4S,5S)-2,2-dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxolane-4,5-dimethanol, (4S,5S)-2,2-dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxolan-4,5-dimethanol, (S,S)-α,α,α’,α’-tetraphenyl-2,2-dimethyl-1,3-dioxolane-4,5-dimethanol, (S,S)-2,2-dimethyl-α,α,α’,α’-tetraphenyl-1,3-dioxalane-4,5-dimethanol, (4S,5S)-2,2-dimethyl-α,α,α’,α’-tetraphenyldioxolane-4,5-dimethanol |
| Molecular Formula | C31H30O4 |
| Molecular Weight | 466.57 |
| InChI | InChI=1S/C31H30O4/c1-29(2)34-27(30(32,23-15-7-3-8-16-23)24-17-9-4-10-18-24)28(35-29)31(33,25-19-11-5-12-20-25)26-21-13-6-14-22-26/h3-22,27-28,32-33H,1-2H3/t27-,28-/m0/s1 |
| InChI Key | OWVIRVJQDVCGQX-NSOVKSMOSA-N |
| Canonical SMILES | CC1(OC(C(O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C |
| Isomeric SMILES | CC1(O[C@@H]([C@H](O1)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US6090950 | Chiral hydride complexes | 2000 |
| US2002/26067 | Catalytic halogenation of activated methylene and methine compounds | 2002 |
| US6184404 | Process for the selective alkylation of aldehydes by means of organozinc compounds | 2001 |
Physical Data
| Appearance | White solid |
| Melting Point, °C | Solvent (Melting Point) |
| 195 – 196.5 | methanol |
| Concentration (Optical Rotatory Power) | Solvent (Optical Rotatory Power) | Wavelength (Optical Rotatory Power), nm | Optical Rotatory Power, deg |
| 1.0 g/100ml | CHCl3 | 589 | -60.6 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
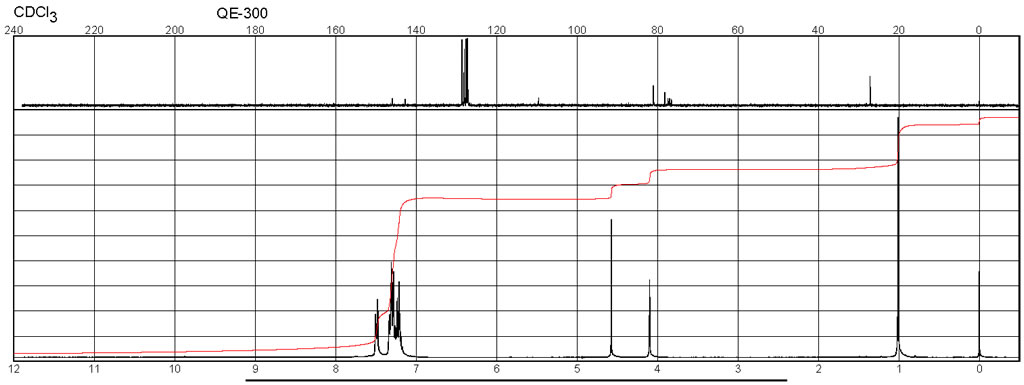
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 |
| Spectrum | 1H | chloroform-d1 | 400 |
| Spectrum | 13C | chloroform-d1 |
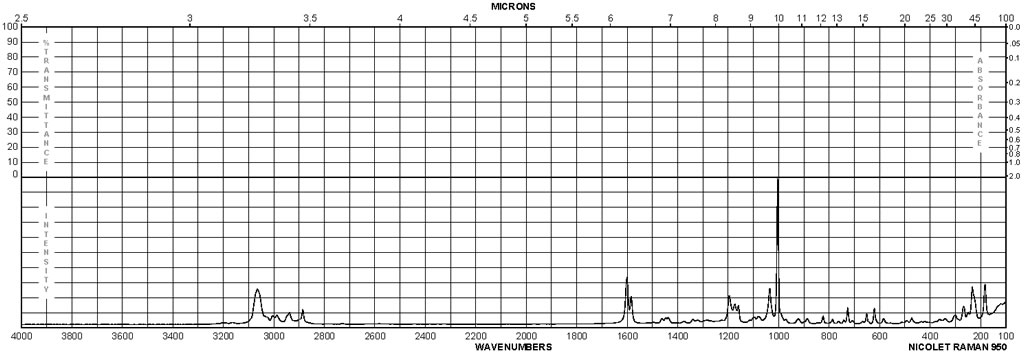
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Peak |
| high resolution mass spectrometry (HRMS), electron impact (EI), spectrum |
Route of Synthesis (ROS)
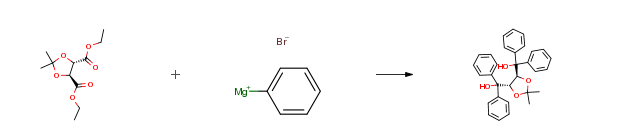
| Conditions | Yield |
| In tetrahydrofuran | 90% |
| In tetrahydrofuran |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| phase transfer catalyst for Schiff’s base alkylation |
| Chiral agent for the asymmetric allylation of alhehydes with allyl bromide in the presence of CrCl2 |
| Use as asymmetric induction Zr catalyst ligand in kinetically controlled Meerwein-Ponndorf-Verley reductions |
| Efficiant catalyst for enantioselective addition of primary alkyl Grignard reagents to aldehydes |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |