| Conditions | Yield |
With 1,4-diaza-bicyclo[2.2.2]octane; tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate In N,N-dimethyl-formamide at 80 – 85℃; for 8h; Time; Heck Reaction;
Experimental Procedure
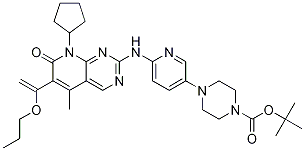
step 1: A mixture of 4-[6-(6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyridino[2,3-d]pyridine-2-yl-amino)-pyridine-3-yl]-piperazine-1-carboxylic acid tert-butyl ester (9.8 g, 0.017 mol), DMF (140 ml), vinyl n-butyl ether (17 g,0.17 mol), K2CO3 (5.27 g, 0.041 mol), Pd2dba3 (1.6 g, 0.17 mmol) and DABCO (0.23 g, 2.04 mmol) were added to the reaction flask, Heated to 80 ~ 85 reaction 8h, The reaction was terminated by TLC, Cooling to 35 deg C, filter, After adding 120 ml of purified water, Crystallization, Filtration, 60 deg C vacuum drying 8h, To give 4-[6-[[6-(1-Butoxyvinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2-yl]amino]pyridin-3-yl]piperazine-1-carboxylic acid tert-butyl ester (9.7 g, Yield 96%). | 96% |
With palladium diacetate; N-ethyl-N,N-diisopropylamine; DPEPhos In methanol; butan-1-ol at 40 – 60℃; for 3h; Temperature; Inert atmosphere;
Experimental Procedure
1 Preparation of a solid form of compound of formula (2)
100 g of compound of formula (3) was suspended in 375 g of dry 1 -butanol and 625 g of dry methanol at 40°C. The suspension was placed under nitrogen and 50 g of compound of formula (4), 50 g of diisopropylethylamine, 1 g of Pd(OAc)2 and 3 g of DPEPhos were added. The reaction mixture was heated at 60°C and stirred at this temperature for 3 hours. (0082) The mixture was filtered, to the fitrate 250 g of methanol and 300 g of water during 40 minutes were added. The suspension was cooled to 0-5°C, solid compound (2) was filtered off and washed with a mixture of 240 g of methanol and 40 g of water. The fitrer cake was dried at 65°C. 94 g of compound of formula (2) was obtained (yield 90% of the theoretical yield) in HPLC purity 99.1%. XRPD pattern of the obtained solid corresponds to XRPD pattern depicted in Figure 1. The content of palladium in obtained solid was 25 ppm. | 90% |
With dichloro(1,1′-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; N-ethyl-N,N-diisopropylamine In butan-1-ol at 95 – 100℃; Inert atmosphere;
Experimental Procedure
Replace the reaction flask with nitrogen, add 1580ml (10V) n-butanol, bubble with nitrogen, add 158g intermediate I, add 108.2g vinyl n-butyl ether, 69.9g N,N-diisopropylethylamine, 7.7g Pd (dppf) Cl2·DCM, nitrogen replacement 3 times, maintaining a slight nitrogen flow, heating to 95-100°C, under nitrogen protection, keeping temperature and stirring, HPLC detection, extraction, concentration under reduced pressure, recrystallization, and drying to obtain the intermediate II 135g, yield 89%. | 89% |
![Route of Synthesis (ROS) of tert-butyl 4-(6-(8-cyclopentyl-5-Methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyrido[2,3-d]pyriMidin-2-ylaMino)pyridin-3-yl)piperazine-1-carboxylate CAS 866084-31-3](https://www.chemwhat.com/wp-content/uploads/2018/10/Route-of-Synthesis-ROS-of-tert-butyl-4-6-8-cyclopentyl-5-Methyl-7-oxo-6-1-propoxyvinyl-78-dihydropyrido23-dpyriMidin-2-ylaMinopyridin-3-ylpiperazine-1-carboxylate-CAS-866084-31-3.png)