tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate CAS#: 571188-59-5; ChemWhat Code: 5890
Identification
| Product Name | tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate |
| IUPAC Name | tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate |
| Molecular Structure | 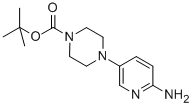 |
| CAS Registry Number | 571188-59-5 |
| Synonyms | OTAVA-BB 1207229; tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, 4-(6-amino-3-pyridinyl)-1-piperazinecarboxylic acid 1,1-dimethylethyl ester, 4-(6-aminopyridine- 3-yl) piperazine-1-carboxylic acid tert-butyl ester, 4-(6-amino-pyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester, 4-(6-aminopyridin-3-yl) piperazine-1-carboxylic acid tert-butyl ester, 4-(6-aminopyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester, 4-(6-aminopyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester, CAS Number 571188-59-5, CAS NO 571188-59-5, CAS 571188-59-5 |
| Molecular Formula | C14H22N4O2 |
| Molecular Weight | 278.350 |
| InChI | InChI=1S/C14H22N4O2/c1-14(2,3)20-13(19)18-8-6-17(7-9-18)11-4-5-12(15)16-10-11/h4-5,10H,6-9H2,1-3H3,(H2,15,16) |
| InChI Key | RMULRXHUNOVPEI-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)(C)OC(=O)N1CCN(CC1)C2=CN=C(C=C2)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP3284746 | PREPARATION AND USE OF KINASE INHIBITOR | 2018 |
| WO2018/51280 | PROCESS FOR PREPARATION OF RIBOCICLIB, ITS ACID ADDITION SALTS | 2018 |
| EP3385262 | PROTEIN KINASE INHIBITOR, PREPARATION METHOD AND MEDICAL USE THEREOF | 2018 |
| WO2017/114351 | CERTAIN PROTEIN KINASE INHIBITORS | 2017 |
| CN106905245 | 2. 4 – disubstituted pyrimidines (by machine translation) | 2017 |
| US2013/116262 | BICYCLIC PIPERAZINE COMPOUNDS | 2013 |
| US2015/210720 | FURO-3-CARBOXAMIDE DERIVATIVES AND METHODS OF USE | 2015 |
| WO2008/7123 | PHARMACEUTICAL COMPOUNDS | 2008 |
| WO2008/32157 | SYNTHESIS OF 2-(PYRIDIN-2-YLAMINO)-PYRIDO[2,3-D]PYRIMIDIN-7-ONES | 2008 |
| WO2004/65378 | 2-AMINOPYRIDINE SUBSTITUTED HETEROCYCLES AS INHIBITORS OF CELLULAR PROLIFERATION | 2004 |
Physical Data
| Appearance | Yellow to brown crystalline powder |
| Density | 1.182g/cm3 |
| Melting Point, °C |
| 124 – 126 |
| 130 – 132 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | chloroform-d1 | 400 | 1H MR (400 MHz CDCl3) δ ppm 7.77 (d, J=2.8, 1H), 7.17-7.14 (dd Jl=9.2, J2=3.2, 1H), 6.49-6.47(d, J=8.0, 1H), 4.24 (br, 2H), 3.56 (t, 4H), 2.97 (t, 4H), 1.48 (s, 9H). |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | 1H NMR (500 MHz, DMSO-de): δ 7.62 (dd, J = 2.99, 0.60 Hz, 1 H), 7.17 (dd, J = 8.85, 2.99 Hz, 1 H), 6.40 (dd, J = 8.85, 0.60 Hz, 1 H), 5.45 (bs, 2H), 3.43 (m, 2H), 2.85 (m, 2H), 1.41 (s, 9H); |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 125 | 13C NMR (125 MHz, DMSO-de): δ 154.8, 153.8, 138.7, 136.8, 125.9, 108.3, 78.9, 50.5, 43.8, 43.0, 28.0; |
| Chromatographic data | Original string |
| LC (Liquid chromatography) | LCMS:279M+H)+, RT=1.120min |
| HPLC (High performance liquid chromatography) | HPLC (Method 2): 6.65 min (99.6percent) (246 nm). |
Route of Synthesis (ROS)
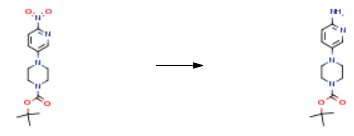
| Conditions | Yield |
| With hydrogen; palladium on activated charcoal In ethanol; water for 2h | 100% |
| With palladium on activated charcoal; hydrogen In ethanol for 3h Experimental Procedure 4- (6-Nitro-pyridin-3-yl)-piperazine-1 -carboxylic acid ie f-butyl ester (2.00 g, 65.9 mmol) and Pd/C (200 mg) in ethanol is stirred with an H2 balloon for 3 h. The reaction mixture is filtered and the filtrate is concentrated under reduced pressure. (0449) Yield: 1 .90 g (quantitative) | 100% |
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃ under 2250.23 Torr for 4h Autoclave Experimental Procedure Step b): synthesis of 4-(6-Amino-pyridin-3-yl)-piperazine-1-carboxylic acid tert- butyl ester formula 4)Step b): A 10 L autoclave was charged with 328.2 g of nitro 1 -Boc-4-(6-nitro-3- pyridyl)piperazine (formula 3) (1 .064 mol, 1 .00 eq.) and 3.5 L MeOH. The system was purged with N2then 22.7 g of 10% Pd/C (50% wet, 21 .3 mmol, 0.02 eq.) were added in one portion. While stirring (300 r.p.m), the reactor was again purged with N2(2 x 2 bar) then pressurized to 3 bar hydrogen. The temperature of the reaction mixture was set to 25°C. IPC of the mixture after 4 h revealed full consumption of starting material. Hydrogen was carefully evacuated from the autoclave and the mixture filtrated. Solids were rinsed with 200 ml MeOH and the filtrate concentrated under reduced pressure. The residue was taken up with 250 ml toluene, concentrated again under reduced pressure and dried at 50°C/15 mbar for 8 h to give 293.5 g of 4-(6-Amino-pyridin-3-yl)- piperazine-1 -carboxylic acid tert-butyl ester (formula 4) as pale violet solid (99.1 % yield).HPLC (Method 2): 6.65 min (99.6%) (246 nm). | 99.1% |
| With hydrogen; palladium 10% on activated carbon In ethanol at 20℃ for 16h Product distribution / selectivity Inert atmosphere | 97% |
| Stage #1: 4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester With iron(III) chloride hexahydrate In ethanol at 80℃ for 0.5h Stage #2: With hydrazine hydrate In ethanol at 80℃ for 14h Concentration | 97.4% |
| With 5%-palladium/activated carbon; hydrogen In ethyl acetate at 42 – 47℃ under 2585.81 Torr Inert atmosphere | 96% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃ under 2250.23 Torr for 12h | 95% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H315: Causes skin irritation [Warning Skin corrosion/irritation] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H402: Harmful to aquatic life [Hazardous to the aquatic environment, acute hazard] H412: Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Market Price | USD 330/kg |
| Use Pattern |
| General chemicals |
| palbociclib intermediate |
| treating hypotrichosis associated with chemotherapy treatment regimens |
| treating hypotrichosis of the eyelashes |
| treatment of dry-eye and related symptoms |
| treating or preventing skin diseases or disorders |
| treating or preventing vitreous adhesions |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
