TPP CAS#: 917-23-7 (20998-75-8); ChemWhat Code: 71635
Identification
| Product Name | TPP |
| IUPAC Name | 5,10,15,20-tetraphenyl-21,23-dihydroporphyrin |
| Molecular Structure | 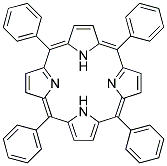 |
| CAS Registry Number | 917-23-7 20998-75-8 |
| EINECS Number | 213-025-9 |
| MDL Number | MFCD00011680 |
| Beilstein Registry Number | 379542 |
| Synonyms | meso-Tetraphenylporphyrin; 5,15,10,20-tetraphenylporphyrin, 5,10,15,20-tetraphenyl-21H,23H-porphine, 5,10,15,20-tetrakis-(phenyl)-21,23H-porphyrin, 5,10,15,20-meso-tetrakis(4-phenyl)porphyrin, 5,10,15,20-tetraphenyl-21H,23H-porphyrin, 5,10,15,20-Tetraphenyl-21H,23H-porphine, 5,10,15,20-tetrakis(phenyl)porphyrinate; 20998-75-8; 917-23-7 |
| Molecular Formula | C44H30N4 |
| Molecular Weight | 614.749 |
| InChI | InChI=1S/C44H30N4/c1-5-13-29(14-6-1)41-33-21-23-35(45-33)42(30-15-7-2-8-16-30)37-25-27-39(47-37)44(32-19-11-4-12-20-32)40-28-26-38(48-40)43(31-17-9-3-10-18-31)36-24-22-34(41)46-36/h1-28,45,48H |
| InChI Key | YNHJECZULSZAQK-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC=C(C=C1)C2=C3C=CC(=C(C4=NC(=C(C5=CC=C(N5)C(=C6C=CC2=N6)C7=CC=CC=C7)C8=CC=CC=C8)C=C4)C9=CC=CC=C9)N3 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP1672961 | COMPOUND, CHARGE TRANSPORT MATERIAL AND ORGANIC ELECTROLUMINESCENT DEVICE | 2006 |
| EP1829871 | ORGANIC COMPOUND, CHARGE-TRANSPORTING MATERIAL, AND ORGANIC ELECTROLUMINESCENT ELEMENT | 2015 |
| US6316493 | Substituted 1,2,4-trioxanes as antimalarial agents and a process of producing the substituted 1,2,4-trioxanes | 2001 |
| US2002/155099 | TRANSFER OF MOLECULES INTO THE CYTOSOL OF CELLS | 2002 |
Physical Data
| Appearance | Blue to purple crystalline powder |
| Solubility | Insoluble in water |
| Melting Point, °C | Solvent (Melting Point) | Comment (Melting Point) |
| 250 | ethanol | |
| 300 | ||
| 450 | benzene | Sublimation.at:>400 degreeC.Zers.. |
| Density, g·cm-3 | Measurement Temperature, °C | Type (Density) |
| 1.276 | 20 | crystallographic |
| Description (Adsorption (MCS)) | Temperature (Adsorption (MCS)), °C | Comment (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Further physical properties of the adsorbed molecule | X-ray diffraction | Ag(111) | |
| Further physical properties of the adsorbed molecule | 26.84 | Cu | |
| Further physical properties of the adsorbed molecule | 24.85 | polarized | toluene |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | aq. buffer, N,N-dimethyl-formamide | 19.99 | bovine serum albumin |
| Association with compound | chloroform-d1 | -32 | 2-phenoxypropionic acid |
| Association with compound | dichloromethane | 9,10-phenanthrenequinone | |
| Association with compound | cyclohexane | 20 | fullerene-C60 |
Spectra
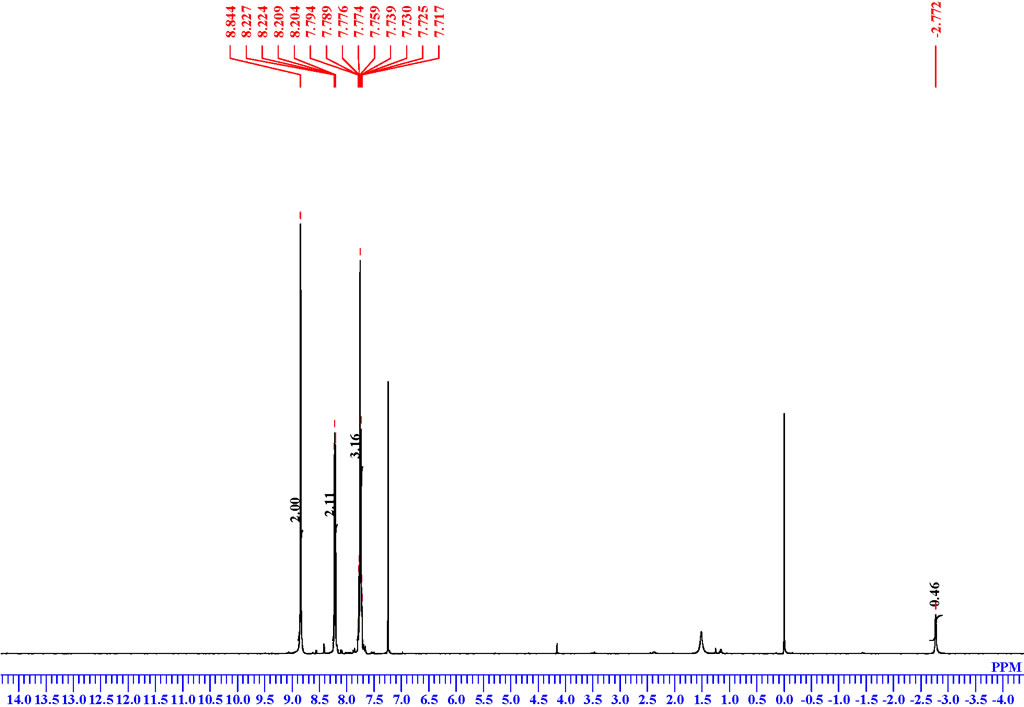
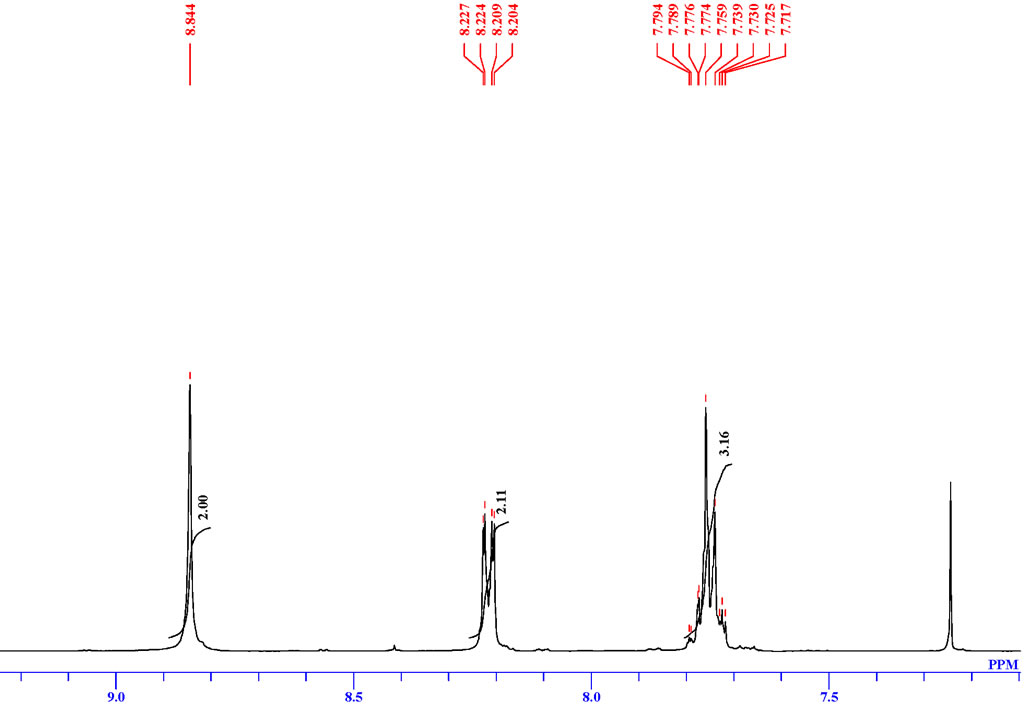
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Description (ESR Spectroscopy) | Solvents (ESR Spectroscopy) | Temperature (ESR Spectroscopy), °C |
| Spectrum | solid matrix | -269.16 |
| Spectrum | nematic phase | -133.2 |
Route of Synthesis (ROS)
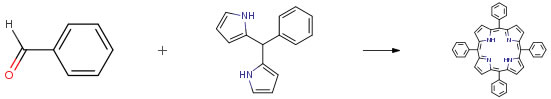
| Conditions | Yield |
| Stage #1: benzaldehyde; 5-phenyldipyrromethane With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 0.5h; Stage #2: With selenium(IV) oxide In dichloromethane at 20℃; for 1h; Experimental Procedure Under room temperature, aryl aldehyde (1 mmol), BF3O(Et)2 (70 μL from a freshly prepared 1 M solution in CH2Cl2) and then pyrrole (70 μL, 1 mmol) were added successively to 10 mL CH2Cl2; reaction was then stirred for 30 min. Finely powdered SeO2 (1670 g, 15 mmol) was sequentially added under vigorous stirring and reaction was then kept for additional 60 min. The resulting mixture was directly filtered through a Celite pad, evaporated on Silica 60 and purified by flash chromatography (Silica 60, 20 .x. 150 mm) with hexanes/CH2Cl2 (2:1) for 1, CHCl3/hexanes (3:2) for 2, CHCl3/hexanes (1:2) for 5, CHCl3/(Et)2O/hexanes (5:2:4) for 3 and CHCl3/hexanes (1:1) for 4 with yields shown in Table 2. Compounds spectrometric properties coincided with literature data (for details, see Supplementary data). | 65% |
| Stage #1: benzaldehyde; 5-phenyldipyrromethane With Indion-130 resin In dichloromethane at 25℃; for 16h; Stage #2: With chloranil In dichloromethane for 3h; Heating; | 24% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, and P501 (The corresponding statement to each P-code can be found at the?GHS Classification?page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 2600/kg |
| Use Pattern |
| Nano-technology |
| in combination with fullerene (C60) |
| organic Field-Effect Transistor (OFET) |
| filter that inhibits transmission of light having a wavelength in the 450+/−50 nm range |
| detecting a cathepsin E expressing cell in vitro or in vivo |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |