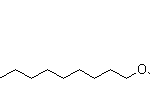
TRANS-7, CIS-9-DODECADIENYL ACETATE CAS#: 55774-32-8; ChemWhat Code: 826163
Identification
| Product Name | TRANS-7, CIS-9-DODECADIENYL ACETATE |
| IUPAC Name | [(7Z,9E)-dodeca-7,9-dienyl] acetate |
| Molecular Structure | 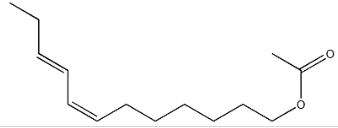 |
| CAS Registry Number | 55774-32-8 |
| EINECS Number | 259-812-0 |
| MDL Number | MFCD00009870 |
| Synonyms | (7Z,9E)-7,9-dodecadien-1-yl acetate, (7Z, 9E)-dodecadien-1-yl acetate, (7Z,9E)-7,9-dodecadienyl acetate, (7Z,9E)-7,9-Dodecadienylacetat, (Z,E)-7,9-Dodecadienyl acetate, (7Z,9E)-dodecadienyl acetate, 7,9-dodecadienyl acetate;CAS Number: 55774-32-8 |
| Molecular Formula | C14H24O2 |
| Molecular Weight | 224.339 |
| InChI | InChI=1S/C14H24O2/c1-3-4-5-6-7-8-9-10-11-12-13-16-14(2)15/h4-7H,3,8-13H2,1-2H3/b5-4-,7-6+ |
| InChI Key | LLRZUAWETKPZJO-SCFJQAPRSA-N |
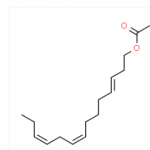
| Canonical SMILES | CC/C=C\C=C\CCCCCCOC(=O)C |
| Patent Information |
| No data available |
Physical Data
| Appearance | Colorless or light yellow oil |
| Solubility | 2.0 mg l-1(20 °C, est.) |
| Flash Point | 61ºC |
| Boiling Point | 309.6±21.0 °C(Predicted) |
Spectra
| TRANS-7, CIS-9-DODECADIENYL ACETATE CAS# 55774-32-8 NMR | 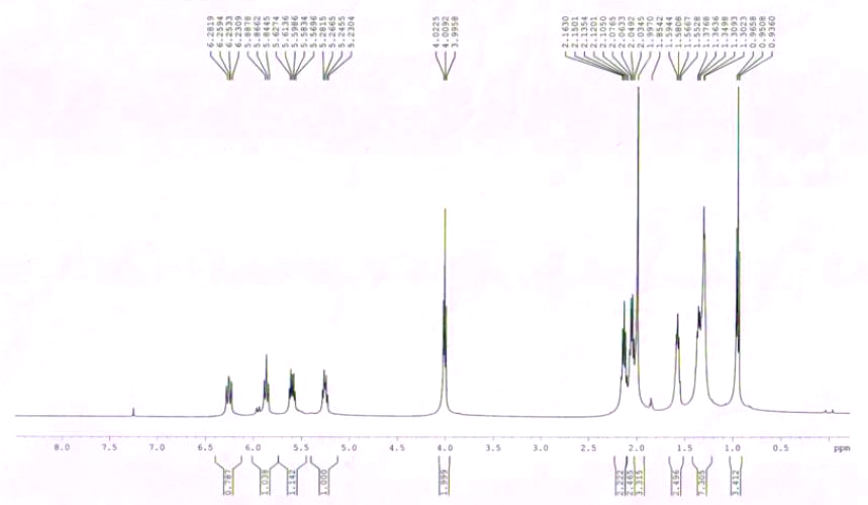 |
| TRANS-7, CIS-9-DODECADIENYL ACETATE CAS# 55774-32-8 CNMR | 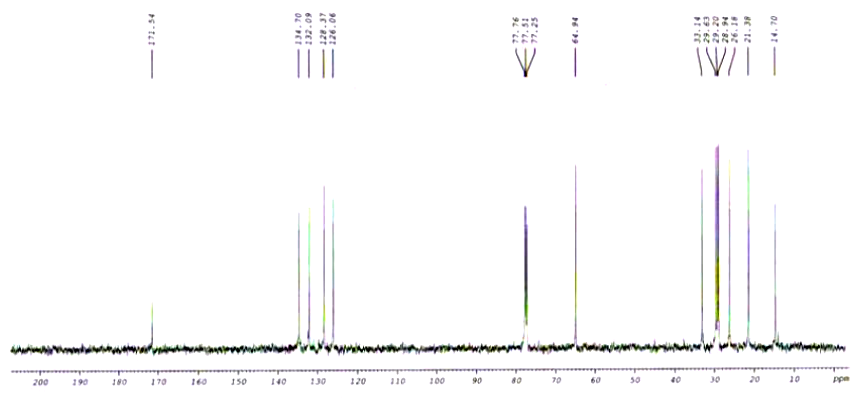 |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), spectrum |
| Spectrum |
| electron impact (EI), spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With dmap; triethylamine at 20℃; for 3h; Overall yield = 43 %; Overall yield = 0.95 g; Experimental Procedure 1.5 Step 5 In a flask, (7E, 9Z) -dodeca-7,9-dien-1-ol (1.60 g) obtained in Step 4 was added.Acetic anhydride (0.99 g),Triethylamine (0.98 g),4-Dimethylaminopyridine (1.1 mg) was added and stirred at room temperature for 3 hours. After adding water and stirring for 30 minutes, t-butyl methyl ether (ml) was added to separate the solution, and the organic layer was washed with water, dried over sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure using an evaporator. Obtained (GC purity 67%, yield 58%). Subsequently, the mixture was purified by silica gel column chromatography (silica gel 60 (68 ml), heptane / ethyl acetate = 10/1), and an isomer mixture containing (7E, 9Z) -dodec-7,9-dien-1-yl acetate (0. 95 g, GC purity 90%) was obtained with a yield of 43%.Isomer ratio is 7Z9E: 7E9Z: 7Z9Z: 7E9E = 12: 36: 41: 11It was. | 99% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (50.56%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H411 (96.11%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | No data available |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 224.343 |
| logP | 5.183 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 10 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| 1 of 1 | Assay Description | Effect : pheromone activity Target : Idaea biselata, geometrid moth Bioassay : delta traps hung ca. 1 m above the ground and set at least 2 m apart; traps hung on the tree twigs or placed in low bushes and checked once a week; red rubber septa as dispensers field trapping tests; in hexane; in the Hagadal and Nasten regions of Uppsala in south-central Sweden from July 4 to August 21, 1993 |
| Results | I. biselata captured: 5 |
| Use Pattern |
| Insect attractant |
| pheromone |
| chemical attractant |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Bio Ltd | https://www.watson-bio.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |


![Structure of 4-[4-(acetyloxy)phenyl]-2-butanone CAS 3572-06-3](https://www.chemwhat.com/wp-content/uploads/2017/11/Structure-of-4-4-acetyloxyphenyl-2-butanone-CAS-3572-06-3-150x89.png)