TRIETHYLGALLIUM CAS#: 1115-99-7; ChemWhat Code: 107461
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| 30962119 | PROCESS FOR THE PREPARATION OF TRIMETHYL METAL COMPOUNDS | 2017 |
| 37457058 | High-purity trialkylgallium and its manufacturing method (by machine translation) | 2015 |
| 26873185 | METHODS OF PRODUCING TRIMETHYLGALLIUM | 2014 |
| 23677215 | PROCESS FOR PREPARING TRIALKYLGALLIUM COMPOUNDS | 2013 |
Physical Data
No data available
| Melting Point, °C |
| -82 |
| -82.3 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 109 | |
| 76 – 78 | 60 |
| 34 – 35 | 10 |
| 34 | 10 |
| 130 – 135 | 650 |
| 142 | |
| 57 – 63 | 256 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.317 | -140.16 |
| 1.307 | -82 |
| 1.331 | -145 |
| 1.0576 | 30 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| GaAs(100) | |
| Adsorption | GaAs(100) |
| Adsorption | N2H4-covered MgO(100) |
| Adsorption | GaAs#dotZn |
| Adsorption | Al/GaAs |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | benzene-d6 | 400.1 | |
| Chemical shifts, Spectrum | 13C | benzene-d6 | 100.6 | |
| 1H | benzene-d6 | |||
| 1H | Cyclohexane-d12 | 25 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Spectrum | neat (no solvent, gas phase) | 400 cm**-1 – 4000 cm**-1 |
| IR |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | gaseous matrix | 190 nm – 390 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium chloride; sodium chloride at 120 – 130℃; under 225.023 Torr; Pressure; Concentration; Temperature; Inert atmosphere; | A 75.9% B n/a |
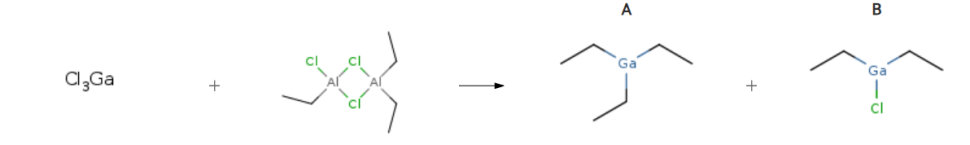
| With potassium chloride; sodium chloride at 120℃; under 225.023 Torr; Inert atmosphere; Experimental Procedure 4 Preparation of triethylgallium (TEG) Example 4 Preparation of triethylgallium (TEG) 68.9 g (0.39 mol) of GaCI3, 32.0 g (0.55 mol, 1 .4 equivalents) of dry NaCI and 17.5 g (0.23 mol, 0.6 equivalent) of dry KCI are placed under argon in a 500 ml four-neck flask provided with stirrer, dropping funnel and a separator maintained at 130°C. While stirring, 120.2 g (0.47 mol, 1 .2 equivalents) of ethylaluminium sesquichloride (Et3AI2Cl3) are added in such a way that the temperature in the reaction mixture does not exceed 120°C. The reaction mixture is subsequently heated while applying a reduced pressure of 300 mbar, and Et3Ga is thus isolated (46.6 g; direct yield 75.9%). | A 75.9% B 19% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H225 (27.54%): Highly Flammable liquid and vapor [Danger Flammable liquids] H250 (100%): Catches fire spontaneously if exposed to air [Danger Pyrophoric liquids] H260 (71.74%): In contact with water releases flammable gases which may ignite spontaneously [Danger Substances and mixtures which in contact with water, emit flammable gases] H261 (27.54%): In contact with water releases flammable gas [Danger Substances and mixtures which in contact with water, emit flammable gases] H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318 (81.16%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P210, P222, P223, P231, P231+P232, P233, P240, P241, P242, P243, P260, P264, P264+P265, P280, P301+P330+P331, P302+P335+P334, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P317, P321, P363, P370+P378, P402+P404, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 156.908 |
| logP | 2.658 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| TRIETHYLGALLIUM CAS#: 1115-99-7 is a commonly used precursor for the production of semiconductor materials such as gallium nitride (GaN) and gallium arsenide (GaAs). These semiconductor materials find critical applications in electronic devices, lasers, and optoelectronic devices. And semiconductor materials derived from triethylgallium can be utilized in the manufacturing of optoelectronic devices such as light-emitting diodes (LEDs), laser diodes (LDs), etc. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |