Trimethylaluminium CAS#: 75-24-1; ChemWhat Code: 34167
Identification
Physical Data
No data available
| Melting Point, °C |
| 15 |
| 15.4 |
| 15.1 |
| 14.5 – 15 |
| 15 |
| 0 |
| 15.2 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 123.5 – 125 | |
| 80 | |
| 125 | |
| 48 – 52 | 20 |
| 129 – 130 | |
| 66 – 68 | 100 |
| 126 | 760 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.7478 | 25 | |
| 0.877 | -10 | |
| 0.752 | 4 | 20 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| Adsorption | AgNO3 |
| TiCl4 activated MgCl2 film | |
| Adsorption | InSb(100) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | tetrahydrofuran-d8, toluene | 400 | |
| Chemical shifts, Spectrum | 1H | tetrahydrofuran-d8, hexane | ||
| Chemical shifts, Spectrum | 1H | benzene-d6, hexane | ||
| Chemical shifts, Spectrum | 1H | benzene-d6 | 26.84 | 400.1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Spectrum | toluene | 2800 cm**-1 – 3700 cm**-1 |
| Bands | toluene | 2800 cm**-1 – 3700 cm**-1 |
| Spectrum | solid matrix | 400 cm**-1 – 4000 cm**-1 |
| Bands | solid matrix | 400 cm**-1 – 4000 cm**-1 |
| Spectrum | further solvent(s) | 400 cm**-1 – 4000 cm**-1 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | gas | 190 nm – 310 nm |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Trimethylaluminium CAS#: 75-24-1
| Conditions | Yield |
| With (1,1′-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate In tetrahydrofuran at 60℃; for 4h; Inert atmosphere; | 98% |
| With palladium diacetate; tris-(o-tolyl)phosphine In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; regioselective reaction; | 89% |
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; triphenylphosphine In tetrahydrofuran at 60℃; for 3h; Inert atmosphere; regioselective reaction; | 83% |
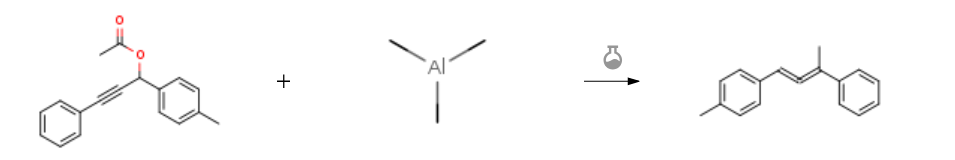
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; triphenylphosphine In tetrahydrofuran at 60℃; for 3h; Inert atmosphere; Experimental Procedure General procedure: Under a dry argon atmosphere, a mixture of Pd(PPh3)2Cl2 (0.0035 g,0.005 mmol), PPh3 (0.0026 g, 0.0100 mmol), and K2CO3 (0.138 g, 1.0mmol) were stirred in THF (2 mL). Then, AlMe3 (0.6 mmol) and propargylacetate (0.50 mmol) were added, respectively. The mixture was stirred for 3-4 h at 60 °C. After completion of the reaction, sat. aqNH4Cl (5 mL) was added and extracted with EtOAc (3 × 15 mL). The combined EtOAc layers was dried (Na2SO4), filtered, and evaporated under vacuum. The crude product was chromatographed on silica gel(hexane or EtOAc and hexane) to afford the corresponding allene product 2 or 5. | 83% |
| With bis(triphenylphosphine)nickel(II) chloride; potassium carbonate; triphenylphosphine In tetrahydrofuran at 60℃; for 6h; Inert atmosphere; | 83% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H250 (98.72%): Catches fire spontaneously if exposed to air [Danger Pyrophoric liquids] H260 (98.4%): In contact with water releases flammable gases which may ignite spontaneously [Danger Substances and mixtures which in contact with water, emit flammable gases] H314 (98.72%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318 (34.29%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P210, P222, P223, P231, P231+P232, P233, P260, P264, P264+P265, P280, P301+P330+P331, P302+P335+P334, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P363, P370+P378, P402+P404, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 72.086 |
| logP | 1.584 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Trimethylaluminium CAS#: 75-24-1 serves as a precursor material in the MOCVD method, used for depositing metal oxide films such as aluminum oxide. And In some organic synthesis reactions, Trimethylaluminium is used as a precursor for catalysts, playing a role in catalyzing certain reactions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |