Triphenyl phosphite CAS#: 101-02-0; ChemWhat Code: 32998
Identification
| Product Name | Triphenyl phosphite |
| IUPAC Name | triphenyl phosphite |
| Molecular Structure | 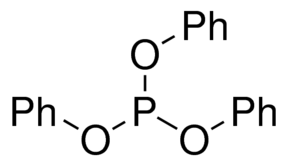 |
| CAS Registry Number | 101-02-0 |
| EINECS Number | No data available |
| MDL Number | MFCD00003032 |
| Beilstein Registry Number | 1079456 |
| Synonyms | triphenyl phosphite, Triphenylphosphite, P(OPh)3;CAS Number: 101-02-0;CAS No.:101-02-0 |
| Molecular Formula | C18H15O3P |
| Molecular Weight | 310.284 |
| InChI | InChI=1S/C18H15O3P/c1-4-10-16(11-5-1)19-22(20-17-12-6-2-7-13-17)21-18-14-8-3-9-15-18/h1-15H |
| InChI Key | HVLLSGMXQDNUAL-UHFFFAOYSA-N |
| Canonical SMILES | c1ccc(cc1)OP(Oc2ccccc2)Oc3ccccc3 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110922427 | Preparation method of sterically hindered alkyl substituted phosphinic acid diesters (by machine translation) | 2020 |
| US2020/185775 | ELECTROLYTE, LITHIUM SECONDARY BATTERY INCLUDING THE ELECTROLYTE, AND METHOD OF MANUFACTURING THE ELECTROLYTE | 2020 |
| CN111233618 | Method for preparing vinyl chloride compound by using phosgene (by machine translation) | 2020 |
| WO2020/161175 | PROCESS FOR THE PRODUCTION OF ACETALS FROM CARBON DIOXIDE | 2020 |
Physical Data
| Appearance | Clear Liquid |
| Solubility | methanol: 25 mg/mL, clear |
| Flash Point | 425 °F |
| Refractive index | n20/D 1.59(lit.) |
| Sensitivity | Air Sensitive & Hygroscopic |
| Melting Point, °C |
| 15.84 |
| 24.84 |
| 20.74 |
| -28.15 |
| 21 – 23 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 360 | 760.051 |
| 183 – 184 | 1 |
| 157 | 0.04 |
| 162 | 0.05 |
| 145 – 147 | 1 |
| 183 – 184 | 1 |
| 161 | 0.2 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5884 | 589 | 20 |
| 1.589 | 589 | 25 |
| 1.59 | 589 | 20 |
| 1.5922 | 589 | 20 |
| 1.5898 | 589 | 35 |
| 1.59106 | 589 | 20 |
| 1.588 | 589 | 25 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.348 | -123.16 | |
| 1.304 | -82.15 | |
| 1.1898 | 4 | 20 |
| 1.1759 | 25 | |
| 1.095 | 4 | 65 |
| 1.164 | 4 | 45 |
| 1.183 | 25 | 25 |
| 1.184 | 18 | 18 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| NMR spectrum of the complex | CDCl3 | -60 | Fe3(CO)12 |
| NMR spectrum of the complex | CH2Cl2 | -92 | Fe3(CO)12 |
| IR spectrum of the complex | CH2Cl2 | 2,2′-biimidazole, Mn(CO)2 | |
| IR spectrum of the complex | CH2Cl2 | 2,2′-bi-1H-benzimidazole, Mn(CO)2 | |
| NMR spectrum of the complex | acetone-d6 | <η5-C9H7Fe(CO)2> | |
| Further physical properties of the complex | toluene | VOCl3 | |
| Further physical properties of the complex | toluene | VCl4 | |
| NMR spectrum of the complex | CDCl3 | 27 | bis(1,1,1,5,5,5-hexafluoro-2,4-pentanedionato)platinum(II) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz | Comment (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 31P | [D3]acetonitrile | |||
| Spectrum | 31P | tetrahydrofuran-d8, water-d2 | |||
| Chemical shifts | 31P | CDCl3 | 162 | ||
| Chemical shifts | 31P | acetonitrile | |||
| Spin-lattice relaxation time (T1) | 31P | temperature dependence. Object(s) of Study: solid | |||
| 2D-NMR | 31P | Second Nucleus: 31P, temperature dependence | |||
| Linewidth of NMR absorption | 1H | temperature dependence | |||
| Spin-lattice relaxation time (T1) | 1H | temperature dependence |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Mid IR (MIR), Bands, Spectrum | neat liquid | -55.16 | |
| Mid IR (MIR), Bands, Spectrum | neat (no solvent, solid phase) | ||
| Bands | 952 – 667 cm**(-1), Einfluss von Loesungsmitteln auf die Lage der IR-Banden. | ||
| Spectrum | 5000 – 465 cm**(-1) |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), spectrum |
| gas chromatography mass spectrometry (GCMS), fragmentation pattern, spectrum |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), spectrum |
| fragmentation pattern |
| chemical ionization (CI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | acetonitrile | |||
| Spectrum | 2,2,4-trimethyl-pentane | 220 – 280 nm | ||
| Absorption maxima | 2,2,4-trimethyl-pentane | 216, 264.4 | 26400, 1900 | |
| Absorption maxima | hexane | 265, 270, 278 |
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Spectrum | temperature dependence | |
| Spectrum | glass | low temperature |
| Spectrum | cryst. | low temperature |
| Low frequency Raman spectrum | temperature dependence. Object(s) of Study: low temperature | |
| Spectrum | neat liquid | time dependence |
| Spectrum | solid |
Route of Synthesis (ROS)
| Conditions | Yield |
| With Hexamethylphosphorous triamide In toluene at 130℃; for 8h; Schlenk technique; Sealed tube; Inert atmosphere; regioselective reaction; | 100% |
| With 1H-imidazole; carbon disulfide; Hexamethylphosphorous triamide In benzene at 20 – 25℃; for 24h; | 86% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class: 9; Packaging Group: III; UN Number: 3077 |
| Under the room temperature and away from light | |
| HS Code | 292029 |
| Storage | Store at room temperature, sealed, keep in a dry place and away from light. |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 310.289 |
| logP | 5.46 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 41.28 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Use Pattern |
| This product is a commonly used chelating agent, widely used in various PVC products. |
| It can maintain the transparency of the product and inhibit the change of color. |
| At the same time, it can increase the antioxidant and light and thermal stability of the main stabilizer. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

