Tris Buffer CAS#: 77-86-1; ChemWhat Code: 135576
Identification
| Product Name | Tris Buffer |
| IUPAC Name | 2-amino-2-(hydroxymethyl)propane-1,3-diol |
| Molecular Structure | |
| CAS Registry Number | 77-86-1 |
| EINECS Number | 201-064-4 |
| MDL Number | MFCD00004679 |
| Synonyms | Trometamol TROMETHAMINE 77-86-1 Tris(Hydroxymethyl)aminomethane Tris Tham 2-Amino-2-(hydroxymethyl)propane-1,3-diol Trisamine Trizma 2-Amino-2-(hydroxymethyl)-1,3-propanediol Tris buffer Tris base Tromethane Tris-base Trisaminol Pehanorm Talatrol Trisamin Trispuffer Tutofusin tris Addex-tham Tris-steril 1,3-Propanediol, 2-amino-2-(hydroxymethyl)- Tris, free base Trimethylolaminomethane Aminotrimethylolmethane Aminotris(hydroxymethyl)methane Tromethanmin Tris (buffering agent) Tris Amino |
| Molecular Formula | C4H11NO3 |
| Molecular Weight | 121.14 |
| InChI | InChI=1S/C4H11NO3/c5-4(1-6,2-7)3-8/h6-8H,1-3,5H2 |
| InChI Key | LENZDBCJOHFCAS-UHFFFAOYSA-N |
| Isomeric SMILES | C(C(CO)(CO)N)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2024/48925 | ORGANIC SALT OF TEREPHTHALYLIDENE DICAMPHOR SULFONIC ACID FOR USE IN UV-BLOCKING COSMETIC COMPOSITIONS, AND PREPARATION METHOD THEREFOR | 2024 |
| WO2023/215521 | HALIDE-FREE AMMONIUM SILANES | 2023 |
| WO2022/34874 | PREPARATION OR COMPOSITION CONTAINING ASCORBIC ACID COMPOUND AND METHOD FOR STABILIZING ASCORBIC ACID COMPOUND | 2022 |
| WO2022/16345 | APPLICATION OF COMPOSITION FOR PLATELET DISAGGREGATION, DISAGGREGATION REAGENT AND DISAGGREGATION METHOD | 2022 |
| WO2022/251191 | ONE-COMPONENT DELIVERY SYSTEM FOR NUCLEIC ACIDS | 2022 |
| WO2021/23645 | CLEAVABLE MULTI-ALCOHOL-BASED MICROCAPSULES | 2021 |
Physical Data
| Appearance | White crystalline power |
| Melting Point, °C |
| 171 |
| 171 – 172 |
| 170.45 |
| 171.1 |
| 170 – 171 |
| 173 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 219 – 220 | 10 |
| Density, g·cm-3 |
| 1.328 |
| 1.226 |
| 1.237 |
| 1.25 |
| 1.313 |
| 1.314 |
| 1.316 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | benzene | benzoic acid | |
| Association with compound | water, ethylene glycol | -196.16 | copper(II) ion |
| Association with compound | water | 25 | icopper(II) ion |
| Stability constant of the complex with … | H2O | 25 | Ca(II) |
| Enthalpy of association | H2O | 25 | Ca(II) |
| IR spectrum of the complex | solid | H3AsO4 | |
| Further physical properties of the complex | solid | H3AsO4 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | water-d2 | 24.84 | |
| Chemical shifts, Spectrum | 1H | |||
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts, Spectrum | 13C | water-d2 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | |
| Spectrum | 1H | dimethylsulfoxide-d6 | 400 | |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Spectrum | 13C |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | |
| ATR (attenuated total reflectance), Bands, Spectrum | |
| Mid IR (MIR), ATR (attenuated total reflectance), Spectrum | |
| Bands, Spectrum | |
| Bands, Spectrum | potassium bromide |
| Spectrum | |
| Bands |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | neat (no solvent, solid phase) | |
| Spectrum | neat (no solvent, solid phase) | |
| Spectrum | H2O | 250 – 500 nm |
| Spectrum | H2O | 320 – 700 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| In methanol at 20℃; | 100% |
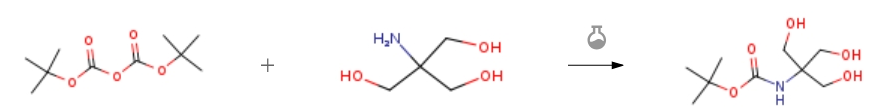
| In methanol; water at 20℃; for 72h; Experimental Procedure A solution of 2-amino-2-(hydroxymethyl)propane-1 ,3-diol (15.7 g, 130 mmol) and di-tert- butyl dicarbonate (31.1 g, 143 mmol) in methanol (400 mL) and water (40 mL) was stirred at ambient temperature for 72 h. The contents of the flask were concentrated under reduced pressure and the resulting white solid was dissolved in minimal hot ethyl acetate and allowed to recrystallise overnight. The crystals were filtered and washed with petroleum ether to give N-(1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)pivalamide (26.5 g, 130 mmol, 100%) as fluffy, white needles. To a solution of N-(1 ,3-dihydroxy-2- (hydroxymethyl)propan-2-yl)pivalamide (9.50 g, 42.0 mmol) and 2,2-dimethoxypropane (16.0 mL, 129 mmol) in DMF (100 mL) was added pyridinium para-toluenesulfonate (0.540 g, 2.15 mmol) at RT. The reaction was stirred at ambient temperature for 15 h. after which time the reaction was complete by TLC (petroleum etheϖethyl acetate, 4:1, visualised with Erlichs). The reaction mixture was diluted with diethyl ether, washed three times with aqueous sodium bicarbonate, once with brine, dried over magnesium sulphate, filtered and concentrated under reduced pressure. The resulting semi-solid was recrystallised from minimal hot petroleum ether to give N-(5-(hydroxymethyl)-2,2-dimethyl- 1 ,3-dioxan-5-yl)pivalamide (7.32 g, 65%) as white crystals. 1H NMR (CDCI3): δ 5.31 (br s, 1H, NH), 4.18 (br s, 1H, OH), 3.85 (d, J = 11.5 Hz, 2H), 3.80 (d, J = 11.5 Hz, 2H), 3.70 (d, 6.6 Hz, 2H), 1.46 (s, 12H), 1.44 (s, 3H). 13C NMR (CDCI3): δ 154.0, 98.8, 80.5, 64.8, 64.5 (2C), 53.4, 28.3 (3C), 26.9, 20.3. . . | 100% |
| With guanidine hydrochloride In ethanol at 35 – 40℃; for 0.0833333h; Experimental Procedure 2.1. General procedure for N-tert-butoxycarbonylation of amines: General procedure: Amine (1 mmol) was added to a magnetically stirred solution of guanidine hydrochloride (15 mol%) and di-tert-butyl dicarbonate (1.2 mmol) in EtOH (1 mL), at 35-40°C and stirred for appropriate time (Table 1). After completion of the reaction (followed by TLC or GC), EtOH was evaporated under vacuum and the residue either was washed with water to remove the catalyst or was dissolved in CH2Cl2 (or EtOAc) and filtered off to separate out the catalyst. Evaporation of the organic solvent (if used in work up) gives almost a pure product. In the cases of using an excess (Boc)2O the product was washed with petroleum ether or hexane to recover the residual (Boc)2O. If necessary, the product was further purified either by crystallization (hexane and dichloromethane, or diethyl ether and petroleum ether) or silica gel column chromatography using EtOAc-hexane (1: 6) as eluent. | 100% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (81.5%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (81.6%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (71.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Store at room temperature for long time, in container tightly sealed ; Protect from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 121.136 |
| logP | -2.662 |
| HBA | 4 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 86.71 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Tris Buffer CAS#: 77-86-1 is a widely used buffer in biochemistry and molecular biology due to its versatile applications including pH Stabilization in Biological Reactions; Electrophoresis; Cell and Tissue Culture; Nucleic Acid and Protein Solubilization; Immunological Assays; Custom Buffer Systems. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |