Uridine CAS#: 58-96-8; ChemWhat Code: 96318
Identification
| Product Name | Uridine |
| IUPAC Name | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione |
| Molecular Structure | 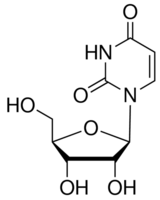 |
| CAS Registry Number | 58-96-8 |
| EINECS Number | 200-407-5 |
| MDL Number | MFCD00006526 |
| Beilstein Registry Number | 754902 |
| Synonyms | uridine, Uridin |
| Molecular Formula | C9H12N2O6 |
| Molecular Weight | 244.2 |
| InChI | InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 |
| InChI Key | DRTQHJPVMGBUCF-XVFCMESISA-N |
| Canonical SMILES | c1cn(c(=O)nc1O)[C@H]2C@@HO)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2019/175633 | REACTIVE, LIPOPHILIC NUCLEOSIDE BUILDING BLOCKS FOR THE SYNTHESIS OF HYDROPHOBIC NUCLEIC ACIDS | 2019 |
| WO2019/126730 | IDOXURIDINE AND ITS ANALOGS AS NEUROPROTECTANS FOR THE TREATMENT OF PARKINSONISM | 2018 |
| WO2017/40892 | ANTI-VIRAL COMPOUNDS | 2017 |
| US2017/44204 | METHOD FOR THE SYNTHESIS OF CLOFARABINE | 2017 |
| WO2016/34735 | CHEMO-ENZYMATIC PREPARATION METHOD FOR PURINE NUCLEOSIDES AND THEIR DEAZA- AND AZA- ANALOGUES | 2016 |
Physical Data
| Appearance | White crystalline powder |
| Solubility | H2O: 50 mg/mL;Soluble in water, dimethylsulfoxide, and methanol. |
| Refractive index | 9 ° (C=2, H2O) |
| Melting Point, °C | Solvent (Melting Point) |
| 164 – 165 | |
| 168 | diethyl ether, hexane |
| 166.5 – 168 | methanol |
| 166.5 – 167 | ethyl acetate, hexane |
| 165 – 166 | ethanol |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.6 | |
| 0.98738 – 0.99879 | 25 – 55 |
| 1.59 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | water | 30.04 | (1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1, 4 ,7,10-tetraazacyclododecane)Zn(ClO4)2 |
| Association with compound | water | 25 | copper(II) ion, 2-(1H-imidazol-4-yl)acetic acid |
| Formation constant of a complex | H2O | 25 | adenosine, Cu(NO3)2 |
| Formation constant of a complex | H2O | 5′-adenosine monophosphate, Cu(NO3)2 | |
| Stability constant of the complex with … | aq. HNO3 | 25 | Cu2+ |
| Stability constant of the complex with … | aq. HNO3 | 25 | acide acetohydroxamique |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | d(4)-methanol | 400 | |
| Chemical shifts | 13C | d(4)-methanol | 100 | |
| Spectrum | 1H | water-d2 | 27 | |
| Spectrum | 13C | water-d2 | 27 | |
| NOESY (Nuclear Overhauser Enhanced Spectroscopy), Chemical shifts | 1H | d(4)-methanol | 24.94 | 750 |
| Chemical shifts, Spectrum | 1H | water-d2 | 37 | 600 |
| Chemical shifts, Spectrum | 1H | d(4)-methanol, water-d2 | 26.84 | 600.2 |
| MAS (Magic-Angle Spinning), Spectrum | 1H | water-d2 | 37 | 500 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Bands | potassium bromide | Ratio of solvents: 66percent | 258 |
| Spectrum | KBr | -253.15 – 26.85 | |
| Bands | KBr | -253.15 – 26.85 | |
| Bands | H2O (pH 4) | 1680 – 765 cm**(-1) | |
| Spectrum | D2O | 2000 – 1250 cm**(-1) | |
| IR |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), time-of-flight mass spectra (TOFMS), spectrum |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), spectrum |
| high resolution mass spectrometry (HRMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), tandem mass spectrometry, spectrum |
| electrospray ionisation (ESI), spectrum |
| IT (ion trap), liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Band assignment, Spectrum | dimethyl sulfoxide, aq. phosphate buffer | 262 | |
| water, sodium hydroxide | 262 | ||
| ethanol | 262 | ||
| methanol | 262 | ||
| Spectrum | water | ||
| Spectrum | alkaline aq. solution, phosphate buffer | ||
| Absorption maxima | acetonitrile, H2O | 262 | 10000 |
| Absorption maxima | acetonitrile, various solvent(s) | 263 | |
| Absorption maxima | 207, 262 |
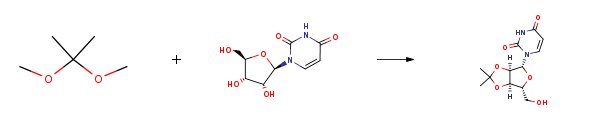
Route of Synthesis (ROS)
| Conditions | Yield |
| With toluene-4-sulfonic acid In acetone for 1h; Reflux; | 100% |
| With toluene-4-sulfonic acid In acetone for 1h; Reflux; | 99% |
| With toluene-4-sulfonic acid In acetone at 60℃; for 24h; Inert atmosphere; | 96% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293359 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 244.204 |
| logP | -2.117 |
| HBA | 8 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 119.33 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target |
| 8 | IC50 | Active | lactosylceramide 4-alpha-galactosyltransferase [Neisseria meningitidis]:Wild | ||
| 7.03 | IC50 | 93 | nM | ||
| 5.7 | concentration (parameter) | 484 | ng/mL | ||
| 5.35 | Km (Michaelis constant)(Michaelis-Menten constant for high-affinity [3H]uridine (5 uM) uptake) | = | 4.5 | μM | Sodium/nucleoside cotransporter 2:Wild |
| 5.01 | IC50 | 9.7 | μM | ||
| 4.89 | Ki (inhibition constant) | = | 13 | µM | Thymidine phosphorylase [human]:Wild |
| 4.64 | IC50 | = | 23 | µM | Solute Carrier Family 29 (Nucleoside Transporters), Member 3 [Leporidae]:Wild |
| 4 | inhibition percentage | 10~90 | % | Adenosine kinase [Mycobacterium tuberculosis]:Wild | |
| 3.92 | IC50 | = | 120 | µM | Integrin alpha-4:Wild |
| Use Pattern |
| Uridine CAS#: 58-96-8 Pharmaceuticals |
| Uridine CAS#: 58-96-8 anti-Enterococcus drug |
| anti-Staphylococcus epidermidis drug |
| uridine-containing compound for pharmaceutical composition |
| diseases of female reproductive organs |
| disease of the retina characterized by excessive angiogenesis |
| rheumatoid arthritis |
| psychiatric disorder |
| mood disorder |
| unipolar depression |
| attention deficit hyperactivity disorder (ADHD) |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | http://ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |