Vibegron CAS#: 1190389-15-1; ChemWhat Code: 1411367
Identification
| Product Name | Vibegron |
| IUPAC Name | (6S)-N-[4-[[(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl]phenyl]-4-oxo-7,8-dihydro-6H-pyrrolo[1,2-a]pyrimidine-6-carboxamide |
| Molecular Structure | 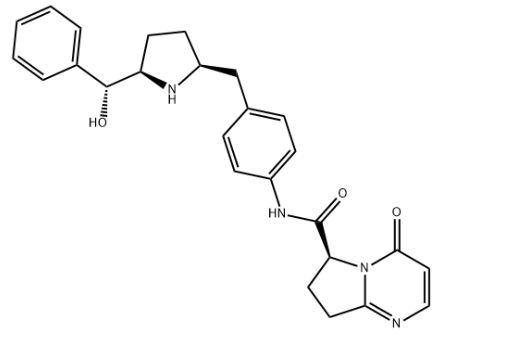 |
| CAS Registry Number | 1190389-15-1 |
| Synonyms | VIBEGRON 1190389-15-1 KRP-114V Gemtesa MK-4618 (S)-N-(4-(((2S,5R)-5-((R)-hydroxy(phenyl)methyl)pyrrolidin-2-yl)methyl)phenyl)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide M5TSE03W5U C26H28N4O3 |
| Molecular Formula | C26H28N4O3 |
| Molecular Weight | 444.5 |
| InChI | InChI=1S/C26H28N4O3/c31-24-14-15-27-23-13-12-22(30(23)24)26(33)29-19-8-6-17(7-9-19)16-20-10-11-21(28-20)25(32)18-4-2-1-3-5-18/h1-9,14-15,20-22,25,28,32H,10-13,16H2,(H,29,33)/t20-,21+,22-,25+/m0/s1 |
| InChI Key | DJXRIQMCROIRCZ-XOEOCAAJSA-N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2009/253705 | HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS | 2009 |
Physical Data
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | dimethylsulfoxide-d6 | 26.84 | 400 |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 26.84 | 500 |
| COSY (Correlation Spectroscopy), Spectrum | 1H, 1H | dimethylsulfoxide-d6 | 26.84 | 500 |
| ROESY (Rotating frame Overhauser Enhancement Spectroscopy), Spectrum | 1H, 1H | dimethylsulfoxide-d6 | 26.84 | 500 |
| HSQC (Heteronuclear Single Quantum Coherence), Spectrum | 1H, 13C | dimethylsulfoxide-d6 | 26.84 | |
| HMBC (Heteronuclear Multiple Bond Coherence), Spectrum | 1H, 13C | dimethylsulfoxide-d6 | 26.84 | |
| Spectrum | 13C | dimethylsulfoxide-d6 | 26.84 | 126 |
Route of Synthesis (ROS)
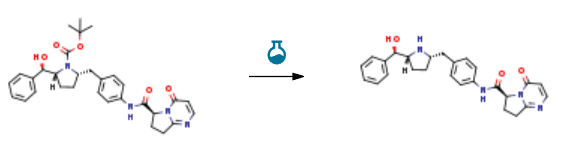
Route of Synthesis (ROS) of vibegron-cas-1190389-15-1
| Conditions | Yield |
| Stage #1: tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate With trifluoroacetic acid In dichloromethane at 20℃; for 1.5h; Stage #2: With sodium hydrogencarbonate pH=8 – 9; | 60% |
| Stage #1: tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate With trifluoroacetic acid In dichloromethane at 20℃; for 1.5h; Stage #2: With sodium hydrogencarbonate pH=8 – 9; | 60% |
Experimental Procedure To a solution of the intermediate from Step A (2.50 g, 4.59 mmol) in dichloromethane (40 ml) was added trifluoroacetic acid (15 ml). The reaction mixture was stirred at ambient temperature for 1.5 h. After removal of the volatiles, saturated NaHCO3 was added to make the PH value to 8-9. The mixture was then extracted with dichloromethane. The combined organic layers were dried over Na2SO4. After concentration, crystallization from methanol/acetonitrile afforded the title compound as a white solid (1.23 g, 60%). 1H NMR (DMSO-d6): δ 10.40 (s, 1H), 7.91 (d, J=6.7 Hz, 1H), 7.49 (d, J=8.3 Hz, 2H), 7.32-7.26 (m, 4H), 7.21 (m, 1H), 7.15 (d, J=8.4 Hz, 2H), 6.23 (d, J=6.7 Hz, 1H), 5.11 (dd, J=9.6, 2.9 Hz, 1H), 5.10 (br, 1H), 4.21 (d, J=7.1 Hz, 1H), 3.20-3.00 (m, 4H), 2.66-2.51 (m, 3H), 2.16 (m, 1H), 1.57 (m, 1H), 1.38 (m, 1H), 1.29-1.23 (m, 2H). LC-MS 445.3 (M+1). |
Safety and Hazards
No data available
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 444.533 |
| logP | 1.581 |
| HBA | 7 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 94.03 |
| Rotatable Bond (RotB) | 7 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 13 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS | |
| 2 of 13 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | AN AGENT FOR TREATING NOCTURNAL POLLAKIURIA | |
| 3 of 13 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Pollakiuria night therapeutic agent | |
| 4 of 13 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Process for preparing beta 3 agonists and intermediates | |
| 5 of 13 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Agent for treating nocturnal pollakiuria | |
| 6 of 13 | Comment (Pharmacological Data) | physiological behaviour discussed |
| Reference | Selectivity and Maximum Response of Vibegron and Mirabegron for β3-Adrenergic Receptors | |
| 7 of 13 | Comment (Pharmacological Data) | physiological behaviour discussed |
| Reference | METHODS OF TREATING HEART FAILURE WITH VIBEGRON |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| Vibegron CAS#: 1190389-15-1 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
